Authors: Tatjana N. Dujsebayeva, Dmitry V. Malakhov, Nikolay N. Berezovikov, Xianguang Guo, Jinlong Liu, and Alexander V. Cherednichenko
The distribution and habitat characteristics of two Eremias species (E. arguta and E. stummeri) from contiguous areas of southeastern Kazakhstan, northeastern Kyrgyzstan, and western Xinjiang, China, were analyzed using GIS modeling. We show that both species are dry-steppe-adapted xerophilous lizards with sclerophilic and partially petrophilic specialization. In spite of the visible similarity of the ecological niches (ENs) occupied by E. arguta and E. stummeri, the latter differs in a number of key variables. While most of the precipitation variables, the radiation of the winter and off-season months, and afternoon (PM) humidity are most important for E. arguta habitats, the habitats suitable for E. stummeri are limited by temperature variables, radiation during most of the year, and morning (AM) humidity.
Differences on this scale indicate the way in which these lizards are adapted to different ecosystems — E. arguta to the plains and E. stummeri to the mountains — and support the views on their different zoogeographical accessory. A visible correspondence of the distributional pattern of the two Eremias species to the mountain piedmonts and low foothills, with Pliocene-Quaternary concreted pebble deposits and the loess strata, permit us to consider mountain trails as the most likely means by which the species expanded in the Late Cenozoic period. Pronounced isolation of the populations in the Northern and the Central Tien Shan intramontane depressions appears as a factor from at least the Late Pliocene (E. stummeri) and the Middle Pleistocene (E. arguta) periods; and the association of the lizard habitats with loess deposits has given rise to the suggestion that there was an absence of serious change in their ranges during the Last Glacial Maximum (LGM). Low precipitation, a high annual temperature range and the high aridity of the climate in general beyond the ENM optimum of E. arguta all point to severe conditions for lizards surviving already in the eastern part of the Ily Basin and explain the limited species distribution in the highly arid deserts of Xinjiang. In terms of methodology, our data highlights the lack of a ‘generalized set’ of BIOCLIM parameters in the ecological niche modeling of the mountain species. In particular, moisture and solar radiation as well as the relief variables play an important role in the ecological health of the poikilothermic xerophilous species inhabiting mountain areas.
Keywords: Eremias arguta; Eremias stummeri; southeastern Kazakhstan; northeastern Kyrgyzstan; western Xinjiang; ecological niche modeling; relief; climate; ecosystem accessory; zoogeographical origin; paleogeography.
PDF-version of article:
INTRODUCTION

Geographical isolation is an important factor influencing biotic evolution; and one which often results in allopatric divergence and speciation (Coyne and Orr, 2004). Geographical isolation is also a widespread and well-demonstrated phenomenon that represents a normal start to reproductive isolation (Grant, 1985). It is well known that the existence of geographical barriers in a broad area greatly favors the formation of races and new species (Mayr, 1966). Central Asia is one such area showing numerous examples of geographic speciation among the plants and animals. The history of the region in the Late Cenozoic period is complex, with the formation of contrast relief, hydrographic network conversion, climate alteration, intensive migration, and the interaction of biota. This led to the origin of many reptilian taxa with limited distribution and specific ecological adaptations (Macey et al., 2005, 2018; Dunayev, 2009; Melville et al., 2009; Solovyova et al., 2018; Zinenko et al., 2015; Orlova et al., 2017; Liu et al., 2014, 2019).
A number of isolated populations of the Eremias species have been found in Central Asia over the past century. Some of them have been justified at the species level, for example, the representatives of the Eremias multiocellata complex (Eremchenko et al., 1992; Eremchenko and Panfilov, 1999a; Orlova et al., 2016, 2017). Some populations have been described as subspecies — Eremias velox roborowskii Bedriaga, 1906 (Bedriaga, “1905” 1907) (deserving recognition at the species level according to recent studies: Liu et al., 2019; Chirikova et al., unpubl. data); E. velox borkini Eremchenko et Panfilov, 1999 (Eremchenko and Panfilov, 1999b), and E. arguta darevskii Tsaruk, 1986 (Tsaruk, 1986).
The main goals pursued by recent researchers are to clarify the taxonomic position and phylogenetic relationships of the forms which have been identified, and then to describe their evolutionary history. In most cases, however, comprehensive data and thus reliable phylogenetic interpretations often suffer from a shortage of representative samples suitable for morphological and molecular analysis (e.g., Guo et al., 2011; Rastegar-Pouyani et al., 2012; Poyarkov et al., 2014). Because most of these forms inhabit the highly dissected areas of Alpine folding, successful geographical sampling has been hindered by the lack of clear knowledge about their distribution.
The above statement accurately describes the cases of two Eremias lizards, E. arguta (Pallas, 1773) and E. stummeri Wettstein, 1940, which inhabit the contiguous territory of Southeast Kazakhstan, Northwest China (Xinjiang) and Northeast Kyrgyzstan. Populations of E. arguta with unusual color spots on the lateral body surface (Fig. 1a) were first identified in the Konyrolen Depression, located in the northwest part of the Ily Basin, and in the Tekes Depression in Southeast Kazakhstan (Dujsebayeva et al., 2007). The following studies revealed the morphological and genetic uniqueness of the “Ily form” of the species (we will in the remainder of this paper refer to this form as species “E. arguta”); and provided a reason for suggesting its taxonomic distinctiveness but unclear status (Orlova et al., 2012; Poyarkov et al., 2014). According to the most recent data, this form is more widely spread in the Ily Basin in both Kazakhstan and Xinjiang (China) than was realized hitherto, and occurs sparsely also in some other intra-montane depressions of the Northern Tien Shan (Poyarkov et al., 2014; Gong et al., 2018).
For a long time, E. stummeri (Fig. 1b) had been considered to be populations of E. multiocellata Günther, 1872 inhabiting the Tien Shan, mainly in Kyrgyzstan and sparsely the extreme southeast of Kazakhstan (Paraskiv, 1956; Zimina, 1959; Yakovleva, 1964). Based on external morphology, Eremchenko et al. (1992) classified this form as a subspecies, E. m. stummeri. Later, through hybridization experiments, they elevated its status to that of a separate species (Eremchenko and Panfilov, 1999a), which has been supported by recent morphological and molecular data (Orlova et al., 2016, 2017). The wide distribution of E. stummeri (under the species name E. multiocellata) in the Issyk-Kul Basin has long been recognized (Shnitnikov, 1928; Zimina, 1959; Yakovleva, 1964). Two older, dubious species records from the Tekes Depression in Kazakhstan (Paraskiv, 1956) were confirmed and, a decade ago, a few sporadic additional records were registered in the Kegen Depression of Kazakhstan (Dujsebayeva et al., 2009). Up to now, therefore, a complete understanding of the distribution of both forms is still lacking.
Recent advances employing geographic information systems (GIS) technology have allowed niche-based modeling of species’ potential distribution, permitting tests of the geographic predictions of suitable habitats in ways not previously possible (Longley et al., 2005). Bearing in mind the issue of the deficiency of representative sampling, we wanted first to summarize the environmental characteristics of known occurrence sites, and to model the fundamental ecological requirements of E. arguta and E. stummeri within the contiguous area shared by Kazakhstan, Xinjiang and Kyrgyzstan by using species distribution modeling (SDM) (Franklin, 2010). This approach has been proven to represent a powerful tool for predicting the geographical distribution of species, especially for those which are rare or characterized by fragmented ranges involving mountains (Suzuki et al., 2007; Doronin, 2012; Tarkhnishvili et al., 2008; Wielstra et al., 2013; Groff et al., 2014; Escalera-Vázquez et al., 2018). Second, to reveal the key variables or environmental drivers underlying species distribution, we undertook a deep comparative analysis. Third, we attempted to determine the optimal ranges for each species and explore the impact of the key variables on species ecology; this brought our research closer to ecological niche modeling (ENM) (Peterson et al., 2011). Conducting this work, we came to certain conclusions concerning the ecosystem accessory of the two species — their zoogeographical origin in relation to the paleogeographical history. Being outside our primary goals, these perspectives appeared to be interesting, especially in term of the potential scope of ENM, and we discuss them as “the notes” in the present paper.
MATERIAL AND METHODS
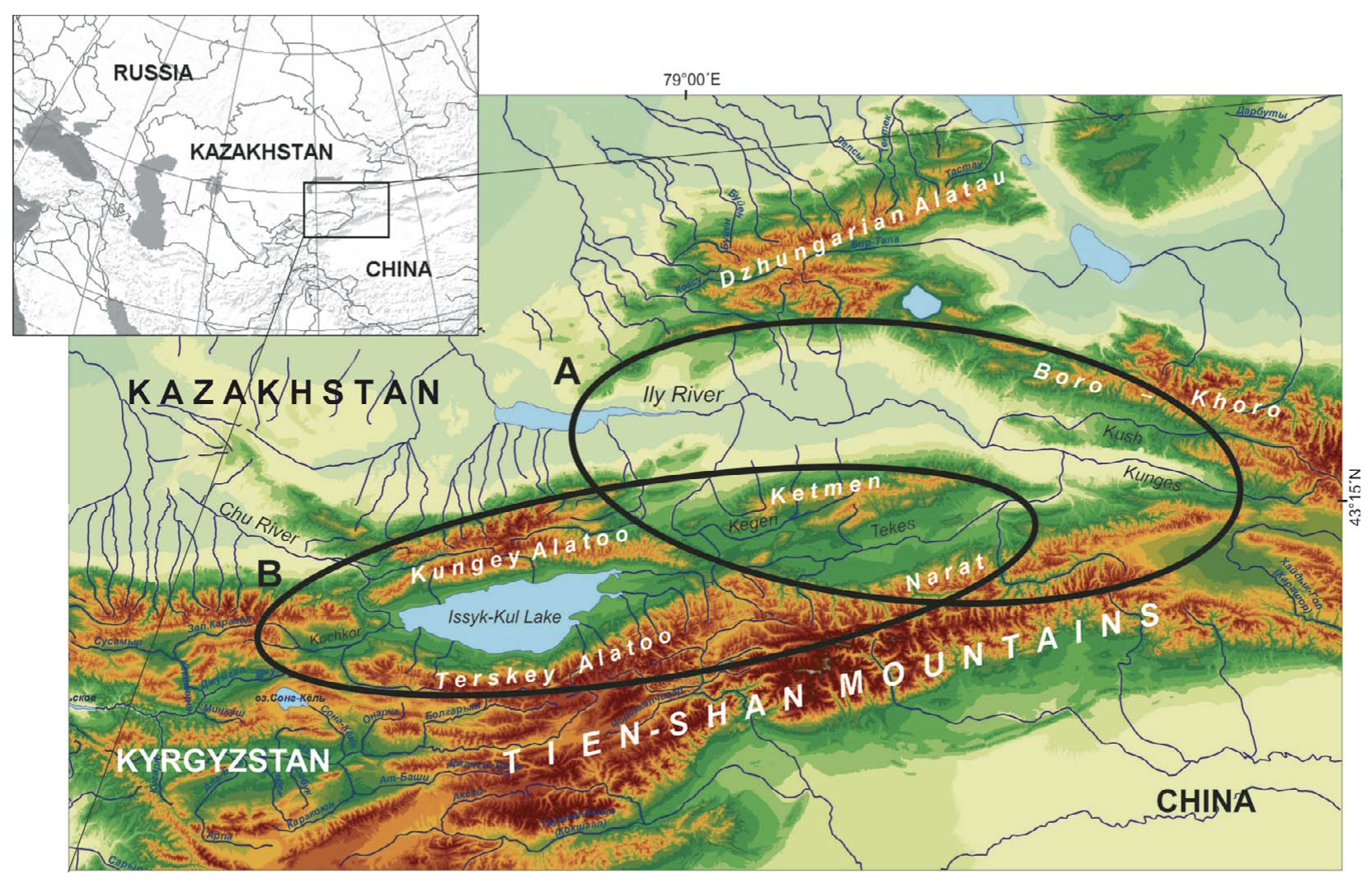
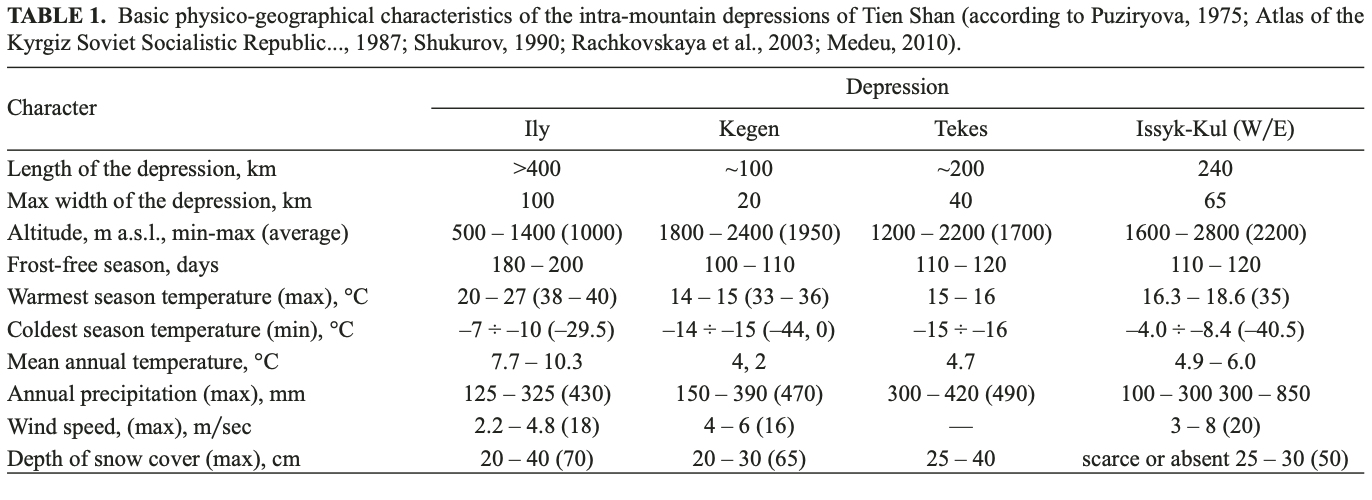
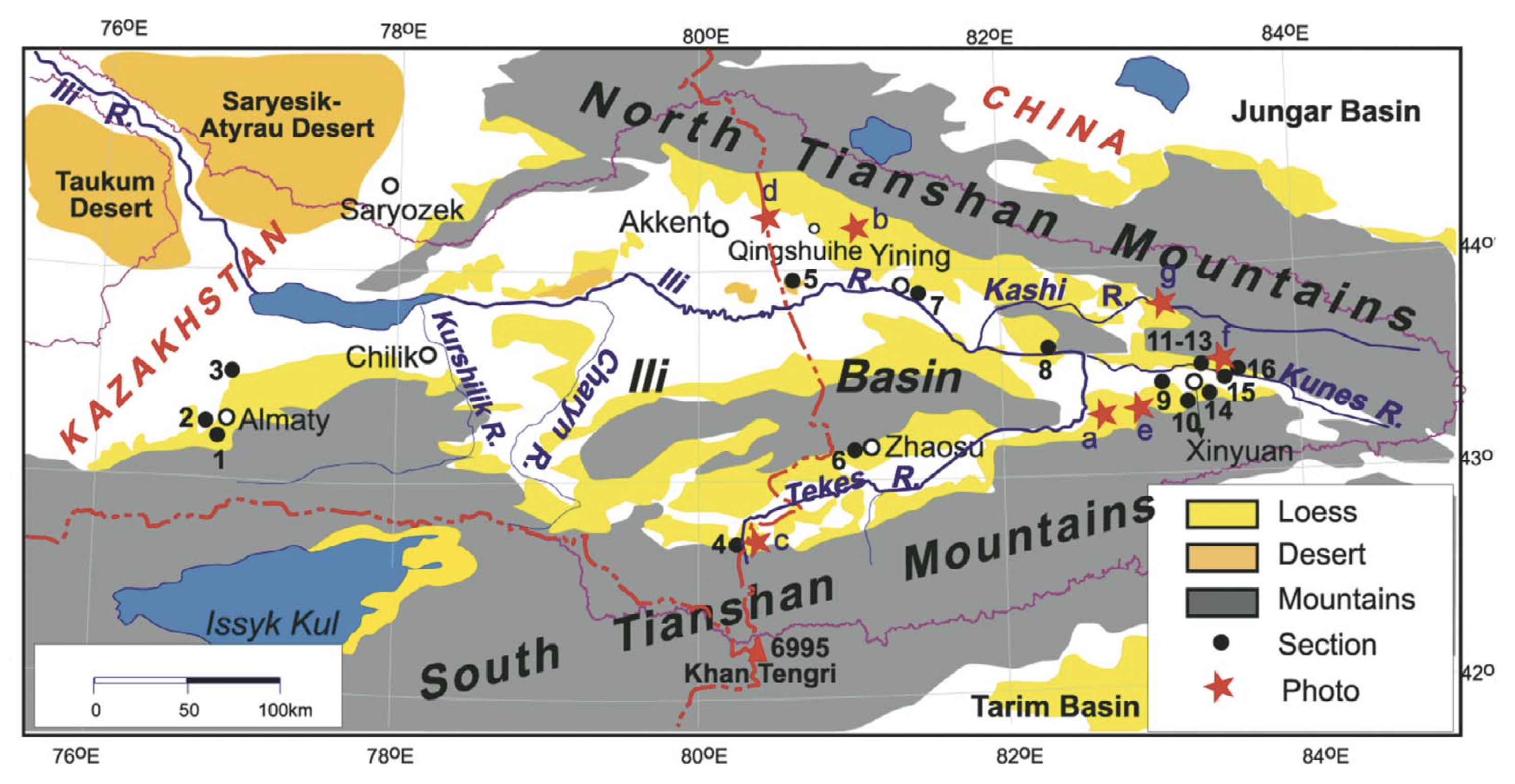
Study area. The area of study includes the intramountain depressions of the Northern, Central and Inner Tien Shan, located mainly within the territories of Southeast Kazakhstan, Northeast Kyrgyzstan, and Northwest China (the Xinjiang Uyghur Autonomous Region, referred to in the remainder of this paper as Xinjiang) which are contiguous to one another, namely, the Ily Basin, the Kegen Depression, the Tekes Depression, and the Issyk-Kul Basin (Fig. 2). These basins belong to structures of Meso-Cenozoic origin, which had a common history of development in the Cretaceous and Neogene (Schultz, 1948; Kostenko, 1978a). Their main physicogeographic characteristics are presented in Table 1.
The Ily Basin is one of the large intra-mountain basins in Central Asia. It is a wide and deep synclinal depression situated between the ridges of Dzhungarian Alatau, Northern and Eastern Tien Shan in the area of southeastern Kazakhstan and Xinjiang (Murzayev, 1966; Visloguzova et al., 1991). The ridges surrounding the basin are not particularly high, on average 1000 – 2000 m and rarely 3000 m a.s.l. (Fig. 2a). The Ily Basin is a complex geomorphological structure consisting of two wide eastern and western flexures and a few small secondary depressions of Pliocene origin — the Konyrolen, Syugaty, and Zhalanash depressions (Fig. 3). The arid landscapes are made up of eolian sands of alluvial and lacustrine origin in the central part of the basin, and by loess and alkali soils on its periphery. The 10 – 20 km wide stony-gravelly plumes with Nanophyton — Salsola plant communities are typical of the mountain piedmonts (400 – 1100 m a.s.l.) (Sokolov et al., 1962; Grigoryeva and Zakharov, 1963). Xerophytic mountain steppes are found at higher elevations (1200 – 1800 m a.s.l.).
The Kegen-Tekes Basin is a wide and long stretched tectonic depression, separating the Northern Tien Shan from the Central Tien Shan. It is located between the Ketmen Ridge and Terskey Alatoo from north to south and between the Kungey Alatoo and Narat Ridge from west to east; and was subdivided into the Kegen Depression and Tekes apparently from the Late Pleistocene (Fig. 2b; Aleshinskaya et al., 1976). Both depressions are located in Kazakhstan, excluding the eastern part of the Tekes Depression which is located in Xinjiang (Fig. 3). Their central areas are occupied by large eponymous mountain rivers with swamped floodplains. Here, meadow landscapes dominate; and only fragments of mountain steppes develop in higher areas.
The Issyk-Kul Basin lies in northeastern Kyrgizstan between the Kungey Alatoo and Terskey Alatoo ranges. These ranges have a maximum height of 4000 – 5000 m. The main feature of the central area of the Issyk-Kul Basin is the Issyk-Kul Lake. The Lake has a maximum depth of 702 m and a surface area of approximately 6000 km2 (Fig. 2b). The level of the Lake fluctuates by around 1607 – 1608 m. The peculiarities of mountain relief mean that there is an uneven distribution of precipitation between the arid western and noticeably wetter eastern sectors of the basin (Table 1). Arid plant communities are seen on the western shores; while cereals and wormwood steppes populate the humid eastern ones. The xerophytic vegetation of the foothills is replaced by boreal forests of Picea schrenkiana tienshanica in the middle altitudes, and alpine meadows across the higher mountain areas. The nival zone with glaciers commences above 3500 m (Shukurov, 1990). Climate warming with a more obvious growth in winter temperature was registered during last two decades for all the intra-montane basins described (Cherednichenko, 2009; Medeu, 2010; Podrezov, 2014).
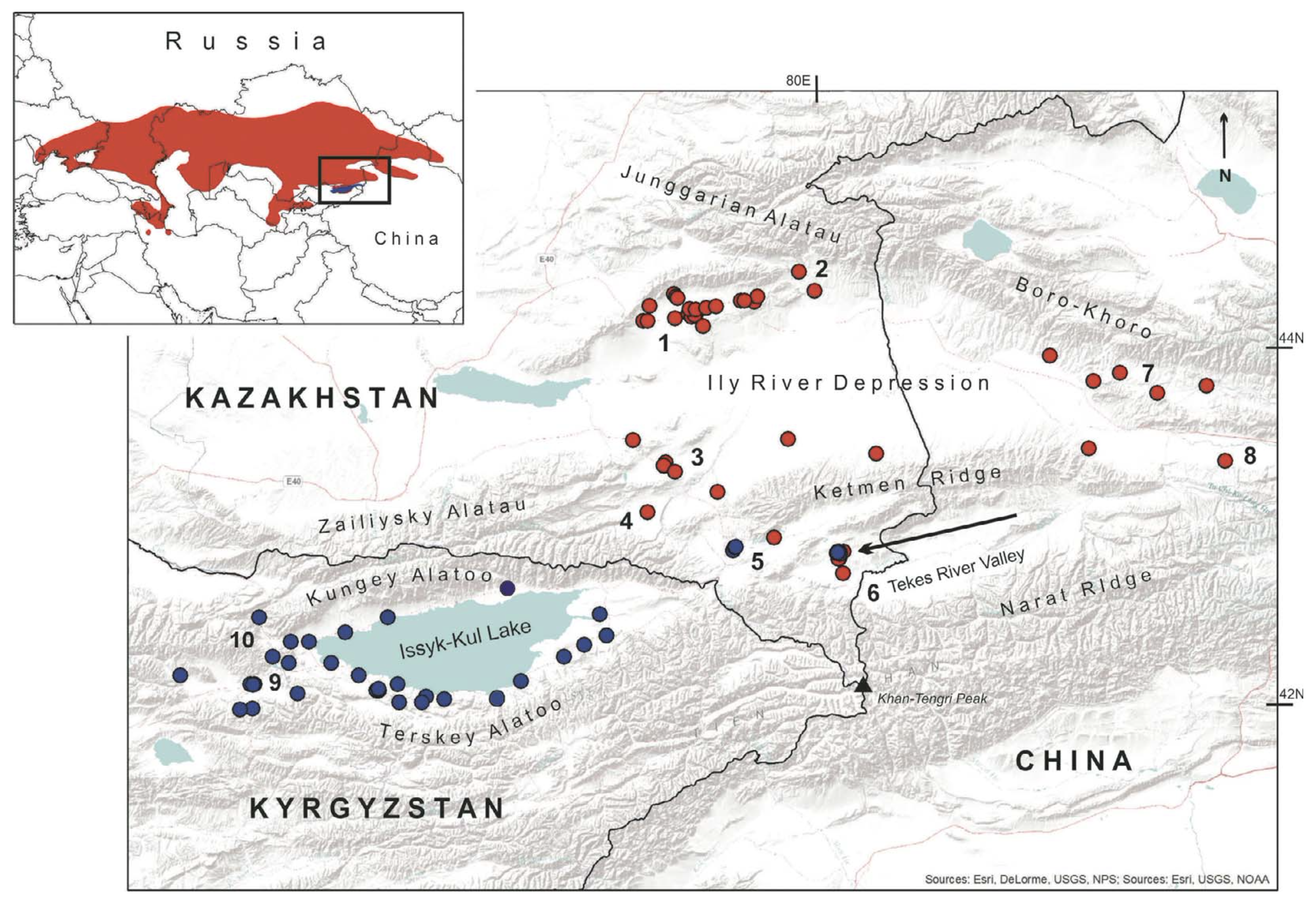
Species locality data. Using the literature (Eremchenko et al., 1992; Eremchenko and Panfilov, 1999a; Sindaco and Jeremèenko, 2008) and our own field data collected in 2006 – 2016 and which has been partially published (Dujsebayeva et al., 2007, 2009), we prepared maps of the records of E. arguta (n = 49) and E. stummeri (n = 41) appearing in the contiguous territory of southeastern Kazakhstan, northeastern Kyrgyzstan and western Xinjiang (Fig. 3). Most of the recent records were GPS-registered, whereas the coordinates of old records were restored using topographic maps of scale 1:200,000 (http:GGsasgis.ruGsasplaneta). Relatively low locality numbers reflect a real spatial rarity of both lizards in the regions studied.
Environmental variables and approach to modelling. The goal of the modeling was to analyze a series of abiotic variables and to determine the envelope of each variable that describes the species prerequisites. A straightforward, original method of modeling, used in this work, was previously described in detail and successfully applied by us for ENM of different animal species as well as fungus (Dujsebayeva and Malakhov, 2017; Malakhov and Chirikova, 2018; Malakhov et al., 2018). We used the following variables: altitude; slope; aspect; curvature (derived from the Digital Elevation Model: http:GGwww. cgiar-csi.orgGdataGsrtm-90m-digital-elevation-database v4-1); monthly and quarterly precipitation and air temperature (BIOCLIM and WORLDCLIM datasets, http:GG www.worldclim.org, http:GGwww.worldclim.orgG BIOCLIM); Global Potential Evapo-Transpiration (Global-PET) Climate Database (http:GGwww.cgiar-csi. orgGdataGglobal-aridity-and-pet-database); relative humidity; and solar radiation (CliMond, https:GGwww. climond.orgGDefault. aspx). In total, using ArcGIS 10.5, we analyzed five relief and 116 climate variables, all of which were represented as rasters with a spatial resolution of approximately 1 km. For each species, we created two models, based on the standard (19) WorldClim Bioclimatic variables (http:GGwww.worldclim.orgG BIOCLIM), and on the expanded set using all the datasets mentioned above.
The paleoclimatic model is principally based on Worldclim 1.4 (http:GGwww.worldclim.orgGversion1) (Hijmans et al., 2005). This dataset includes past climatic conditions (downscaled global model output) and future conditions as well (downscaled global climate model (GCM) data from CMIP5 (IPPC Fifth Assessment). In the present paper, we used the Last Glacial Maximum (LGM) set (about 22,000 years ago). The ranges of key variables, obtained from the analysis of recent conditions, were applied to the datasets of past conditions, assuming the principle of actuality, i.e., the species prerequisites may not change significantly over time, so the optimal range of any variable is considered actual for the recent, distant past and the future.
The geographical descriptions, classifications and terminology used in the present paper are based on the following resources: relief (Sharaya and Shary, 2004; Blaga, 2012); geomorphological and sediment division (Kostenko, 1970; Medeu, 2010); and climate (Kottek et al., 2006; Peel et al., 2007; Podrezov, 2014). The locality names are taken from http:GGsasgis.ruGsasplaneta.
RESULTS
Distribution of Eremias arguta and E. stummeri in the contiguous territories of Kazakhstan, Xinjiang, and Kyrgyzstan based on field data
During 2013 – 2016, we recorded additional occurrences of the “Ily form” of E. arguta in the inter-mountain depressions of the Northern and Eastern Tien Shan within the territories of both Kazakhstan and Xinjiang. The lizards were found in the Usek River Valley (on the southern slopes of Dzungarian Alatau); in the Zhalanash and Suygaty depressions; in the northern and southern piedmonts of Ketmen Ridge; along the right bank of the Charyn River; in the Zhabyr and Aybirzhal areas within the Tekes Depression; and in the Kash and Kunges river valleys (Fig. 3).
In Kazakhstan and Xinjiang, E. arguta habitats were placed in the periphery of the wide Ily Basin and sparsely scattered in the intermontane depressions of the Northern and Eastern Tien Shan at 630 – 2200 m. Their habitats were characterized by hard clay loess soils, often covered by abundant smaller and larger stones, gravel and pebbles with 30 – 50% vegetation from Krascheninnikovia ceratoides, Festuca valesiaca, Nanophyton erinaceum, Artemisia sp., and Achnatherum sp. as the dominant species (Fig. 4a, b). Rarely, bushes of Caragana sp., Atraphaxis compacta, and A. frutescens grew in mosaic pattern along the dry riverbeds which cross the piedmont and foothills (Fig. 4c). In the extreme southeast of the range, the lizards occurred in the non-typical hilly areas. The lizards did not avoid the areas of cattle grazing with secondary weed plant associations but were absent in the central part of Ily Depression and in small sands near Kegen and
Karasaz villages (Kegen Depression) covered with aeolian sands. Our field inspection did not reveal any new localities for the species in Kazakhstan; but it occurred more densely within its range than was apparent before (Fig. 3).
The habitats of E. stummeri in Kazakhstan and Kyrgyzstan were mainly located in the mountain valleys and, to a lesser degree, the piedmonts and foothill slopes. In the Issyk-Kul Basin, E. stummeri was located at 1609 – 2100 m along the lake shores for almost the entire circumference (Fig. 4d); but was more abundant in the western and southwestern sectors, which were characterized by desert landscapes. The lizard preferred the dry and desert-like steppe landscapes with groups of Festuca–Artemisia or Artemisia; and often with a large proportion of rubble. It was also seen on the foothills with scarce xerophyte vegetation. However, it avoided the typical gravel deserts with scarce Artemisia–Salsola vegetation which are a partial feature of the west of the Issyk-Kul Basin, as well as the high and humid meadow areas in the east (Zimina, 1959).
In the Kegen and Tekes depressions, E. stummeri inhabited the beds of dry streams with steep banks; deep clay ravines; or the clay and gravel trails of hilly mountains covered with Festuca, Artemisia, Stipa, and Phleum species and, rarely, bushes of Spirea, Rosa, and Lonicera (Fig. 4e). We found a number of habitats in Neogene clay outcrops (Zhabyrtau and Sholadyr ridges). No lizards were found in the small and unique areas of relict sands near the Kegen and Karasaz villages in the Kegen Depression. The only record in the contiguous area of Xinjiang originated in the right-bank part of the upper reaches of the Tekes River Valley. Few lizards were found in areas of pasture with loess soil and quite dense vegetation, the latter dominated by Achnatherum sp. The lizards were absent from the wide area of the swampy valleys of the Kegen, Karkara, and Tekes rivers within the Kegen and Tekes depressions.
In the area under examination, the maximal altitudes of the habitats of E. arguta and E. stummeri reached 1900 – 2200 m; and were recorded in the southern trail of Zhabyrtau Mountains where the two species lived sympatrically and syntopically (Fig. 3, arrow; Fig. 4f). They inhabited the same clay and gravel xerophytic plains with sparse Achnatherum sp. and located along the ridges of hills; and had shelters under contiguous stones or bushes. In dirt-filled roadside ravines with denser growth, we encountered the third species — Lacerta agilis Linnaeus, 1758.

Ecological Niche Modeling of Eremias arguta and E. stummeri habitats by abiotic variables
Relief. The optimal ranges of the orographic variables outline a suitable relief for E. arguta habitats which is almost flat or possesses insignificantly convex and slightly sloping surfaces, at a height of 1058 – 1839 m a.s.l. (Table 2). Eremias stummeri habitats were found at higher altitudes (1635 – 2030 m a.s.l.); and had a greater curvature and were more sloping (Table 2). Unlike E. arguta, E. stummeri did not show a clear preference for aspect; and was absent from only the most northern slopes. Slope, curvature and aspect were the key variables for E. stummeri ENM (Fig. 5).
The variables of the orographic model and the field observations point to the habitats of E. arguta and E. stummeri being associated with different types of mountain mesorelief. E. arguta habitats adhered to the flat or slightly sloping mountain piedmonts of the peripheral mountain ridges (Fig. 2a); while E. stummeri penetrated far into the higher reaches of the mountain systems, where it occupied the sloping peripheries of the intra-montane depressions and the river valleys between the mountain ridges (Fig. 2b).
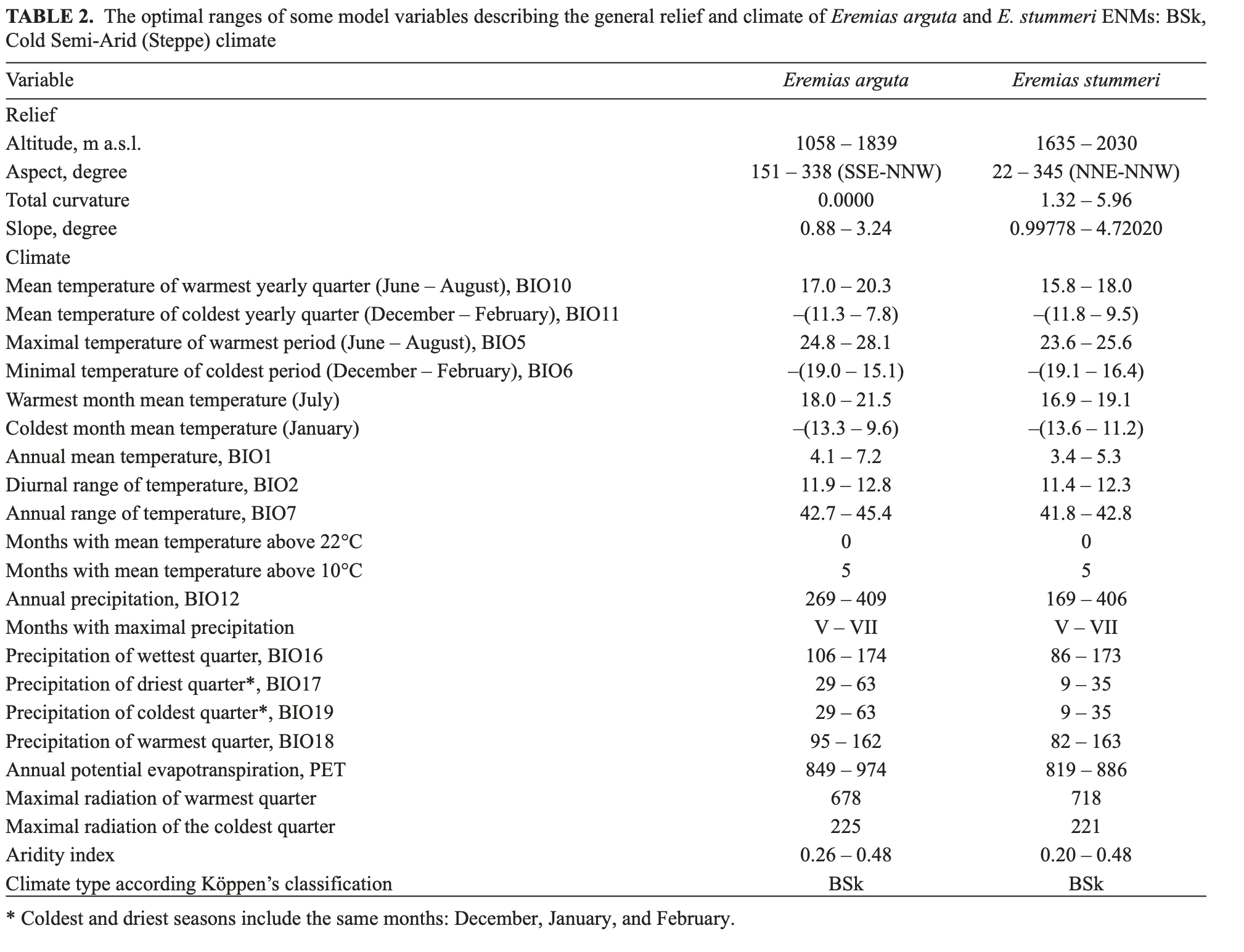
Climate. Temperature. In terms of the thermal parameters, the areas suited to the habitats of E. arguta and E. stummeri stretched out along the piedmonts and low foothills of the Dzhungarian Alatau and the Northern Tien Shan in a relatively narrow zone. Eremias arguta appeared to be somewhat more thermophyle than E. stummeri. Excluding off-seasons, the minimum, mean and maximum temperatures of the habitats of E. arguta exceeded those of E. stummeri by 0.5 – 2.0°C (Table 2). That difference was more prominent in summer than in winter.
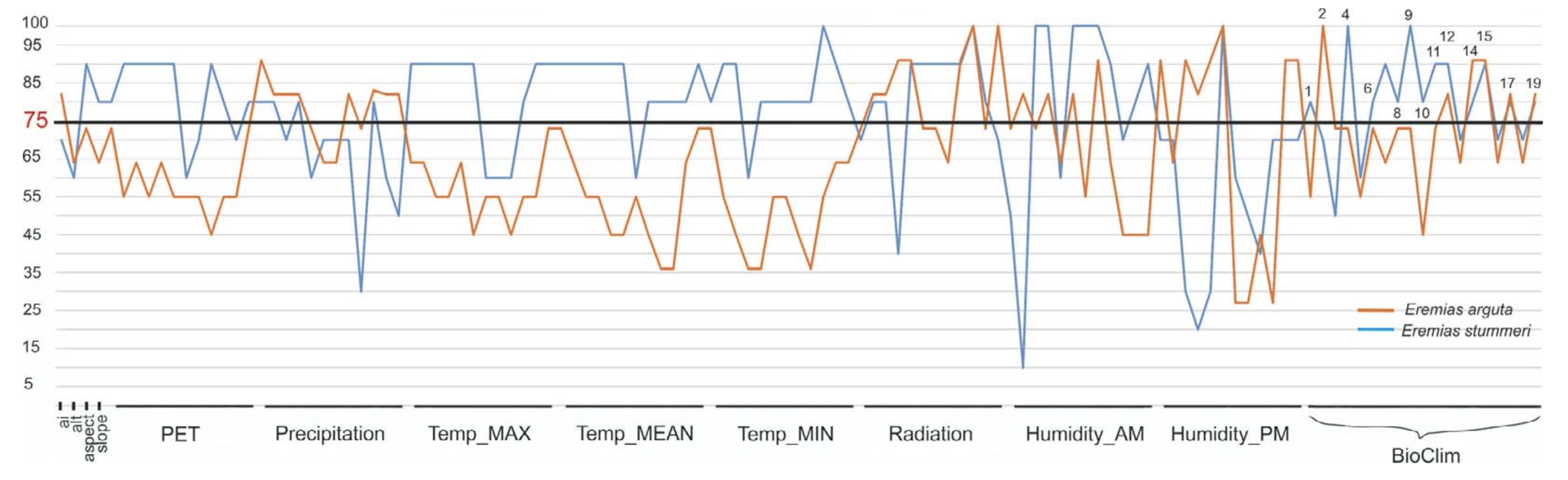
Only the mean temperature diurnal range (BIO2) from the temperature set was included in the key variables of E. arguta ENM; while a great number of temperature variables had special importance for E. stummeri ENM (Table 2, Fig. 5). These are the mean and minimum temperatures for almost all months of the year, the maximum temperatures for winter and off-spring seasons, and eight indexes from the BIOCLIM set (BIO1, BIO4, BIO6 – 11: Table 2, Fig. 5).
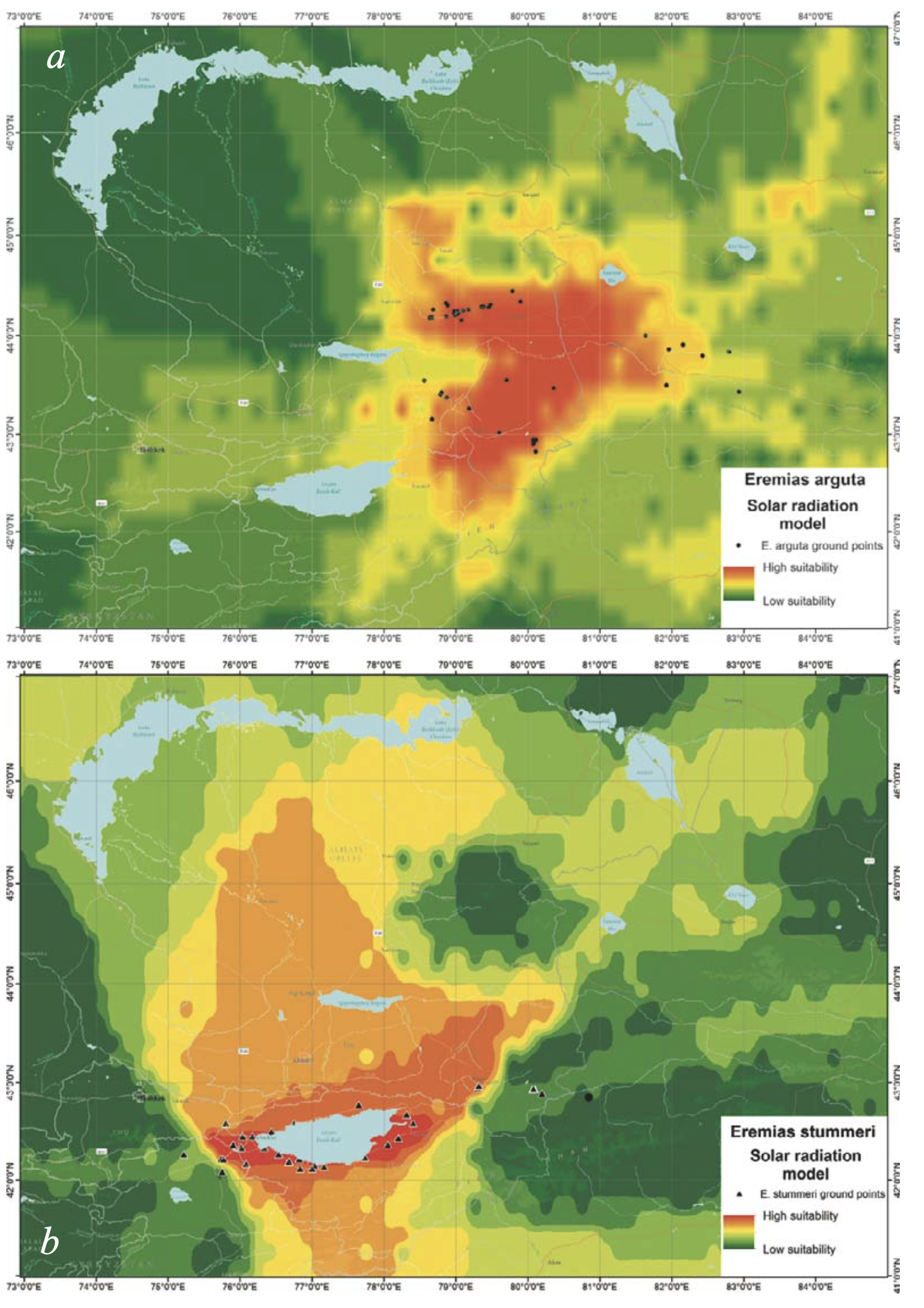
Radiation. During the summer season, the habitats of E. stummeri received slightly more total solar radiation in comparison with the habitats of E. arguta (718 against 667 kkalGcm2Gmin, respectively). During the winter, incoming solar radiation was almost similar (221 against 225 kkalGcm2Gmin: Table 2). However, the spatial distribution of suitable radiation differed significantly between the species. Incoming solar radiation was uniform within the range of E. arguta (Fig. 6a) while it differed by heterogeneity in the range of E. stummeri (Fig. 6b). The most suitable conditions occurred in the southern part of the Issyk-Kul Basin. Those in the northern part of the Issyk-Kul Basin and Kegen Depression were less suitable. The lowest suitability was found in the Tekes Depression of both Kazakhstan and Xinjiang territories and also in the Kochkor Valley of Kyrgyzstan. Radiation throughout most of the year was important for both species, except for the summer months, which were more significant for E. stummeri (Fig. 5).
Precipitation. In the wider range of annual precipitation for E. stummeri, ENMs of both species were characterized by distribution of precipitation throughout the year, with the maximum in the late spring-summer season (May-July) and the minimum in winter (December – February) (Table 2). In general, E. arguta ENM showed 2 – 12 mm higher precipitation per month compared with E. stummeri especially in the winter and off-seasons. The total sum of the winter precipitation differed twice between the species, while the summer precipitation was almost similar (Table 2).
The highest suitability of habitat for E. arguta was found in the piedmonts and low foothills of the northern and southern slopes of Dzhungarian Alatau; the northern slopes of the Northern Tien Shan ridges (Zailiysky Alatau, Kungey Alatau, and Ketmen) in Kazakhstan; the upper section of the Borotala River Valley in Xinjiang; and the Kochkor Valley and Son-Kol Lake Basin in Kyrgyzstan. The central part of the Ily Basin had a lower suitability and the Xinjiang part of the species range was almost unsuitable (Fig. 7a). For E. stummeri, the most suitable habitats were distributed south of those of E. arguta in the Northern, Inner, Central and Eastern Tien Shan, as well as in the edge of the mountainous area in the Dzungarian Depression (Fig. 7b).
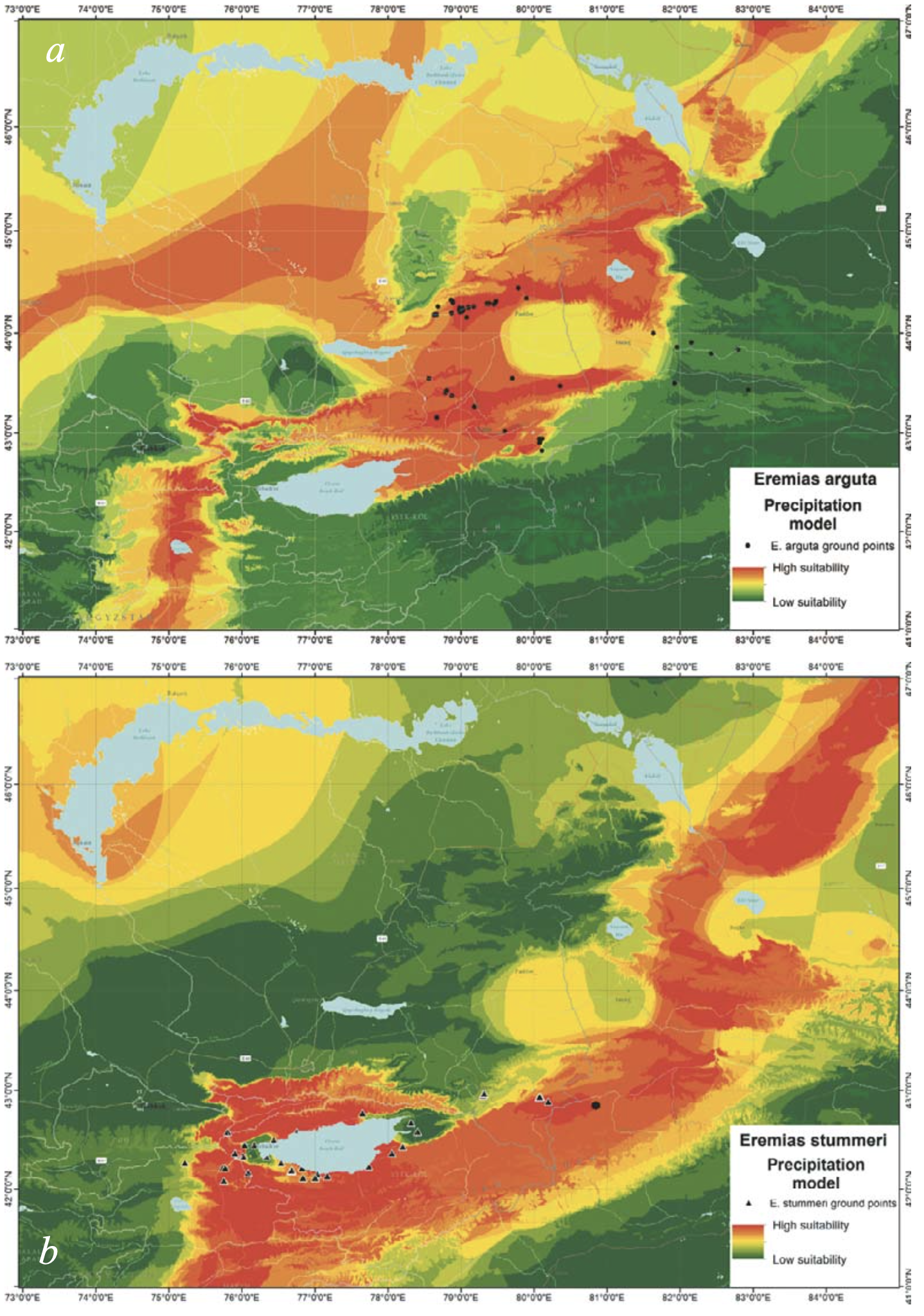
As can be seen from Fig. 7, the territories suitable for the two species in terms of precipitation did not overlap, and that difference was caused by mainly by winter precipitation, especially in December (Fig. 8). Habitat suitability for E. arguta depended more upon precipitation than for E. stummeri. Precipitation across eight months was important for E. arguta ENM, as opposed to four months for E. stummeri. The cold months (with a higher value for E. arguta) were among the most important for the ENMs of both species. The two species were almost the same in terms of the BIOCLIM key variables, except for BIO13 (precipitation in the wettest month) which is important for E. arguta (Table 2).
Potential evapotranspiration. The ENMs of both species were characterized by the visible predominance of the annual PET over annual precipitation (almost 2 – 3 and 2 – 5 times, respectively, Table 2). In the coldest quarter of the year (December – February), PET and precipitation were close to equilibrium in E. stummeri ENM; but PET exceeded precipitation less significantly in E. arguta ENM. For both species, PET was minimal in January and maximal in July. E. arguta ENM had a higher PET during most of the year compared with E. stummeri; but it was most significant (up to 6 – 13 mm) during the warm season (March – August) only. There was no one PET variable among the key parameters of E. arguta ENM habitats while PET for most months was important for E. stummeri (Fig. 5).
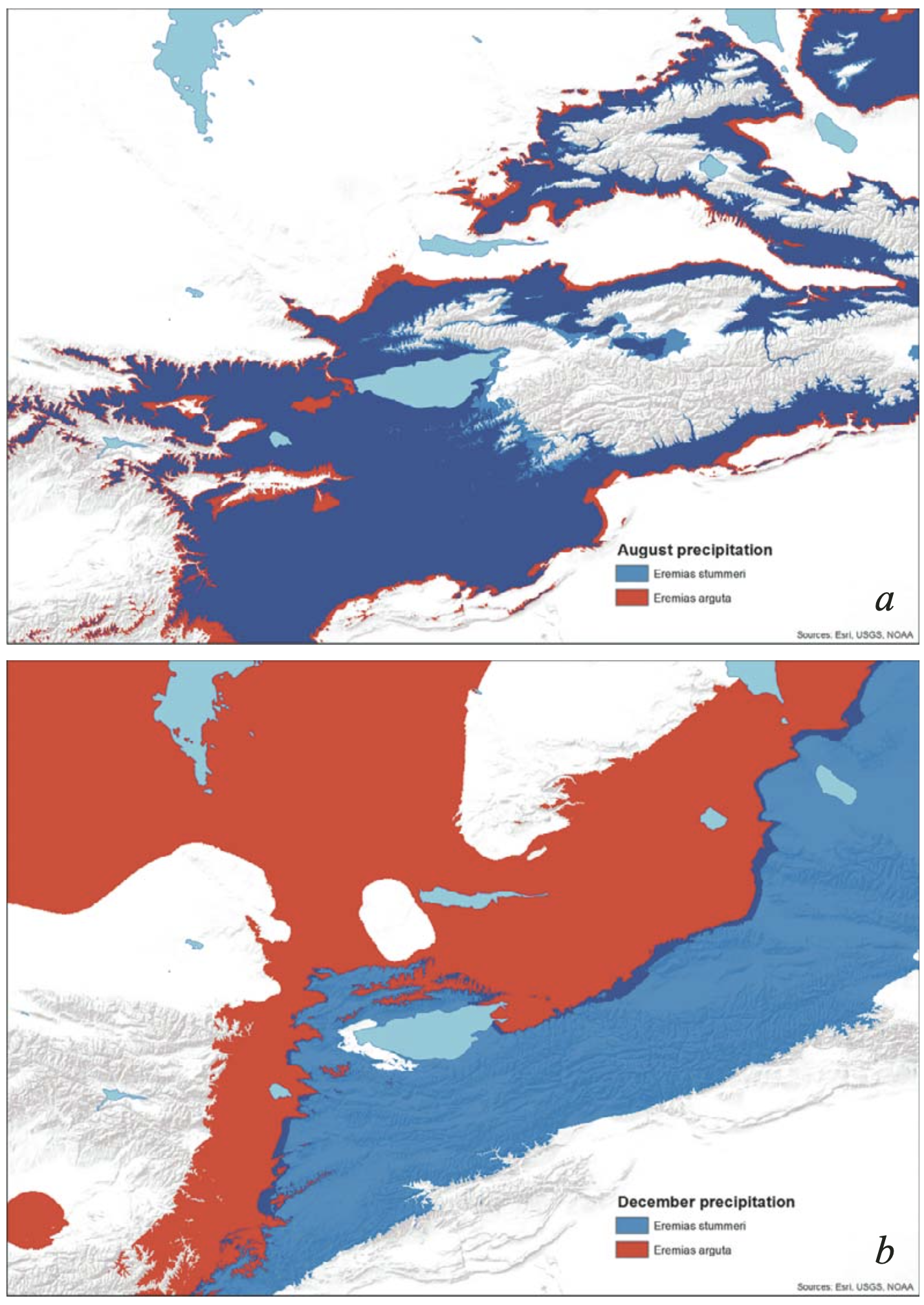
Inter-species comparison has shown that patterns of annual precipitation and PET did not differ significantly between the two species during the warmer half of the year. However, with a visibly similar PET during the colder half of year (September – April), the habitats of E. arguta were characterized by precipitation levels two to four times higher than that of E. stummeri.
Relative Humidity. The ENM of E. arguta differed in the uniform and continuous distribution of available relative humidity at maximal suitability in the Zhalanash and Kegen depressions, the northern foothills of Ketmen and the eastern sector of the Issyk-Kul Basin (Fig. 9a). Less suitability was shown for the western part of the IssykKul Basin and the central part of the Ily Basin. The lowest suitability for humidity was found in the Xinjiang part of the species’ range, except for the Tekes River Valley.
The most suitable humidity for E. stummeri appeared to be in the Issyk-Kul Basin; partially along the foothills of Zailiysky Alatau; and in the Naryn River Valley (Kyrgyzstan), inhabited by the closest species of the E. multiocellata complex E. szczerbakii Eremchenko et Panfilov, 1999 (Fig. 9b). Unlike the E. arguta, the distribution of suitable humidity for E. stummeri had a less uniform pattern.
The health of E. arguta habitats depended more upon postmeridian (RHPM) than antemeridian (RHAM) humidity (seven and four months respectively). Antemeridian humidity was more important for E. stummeri (eight months) (Fig. 5).
Distribution of Eremias arguta and E. stummeri by GIS modeling
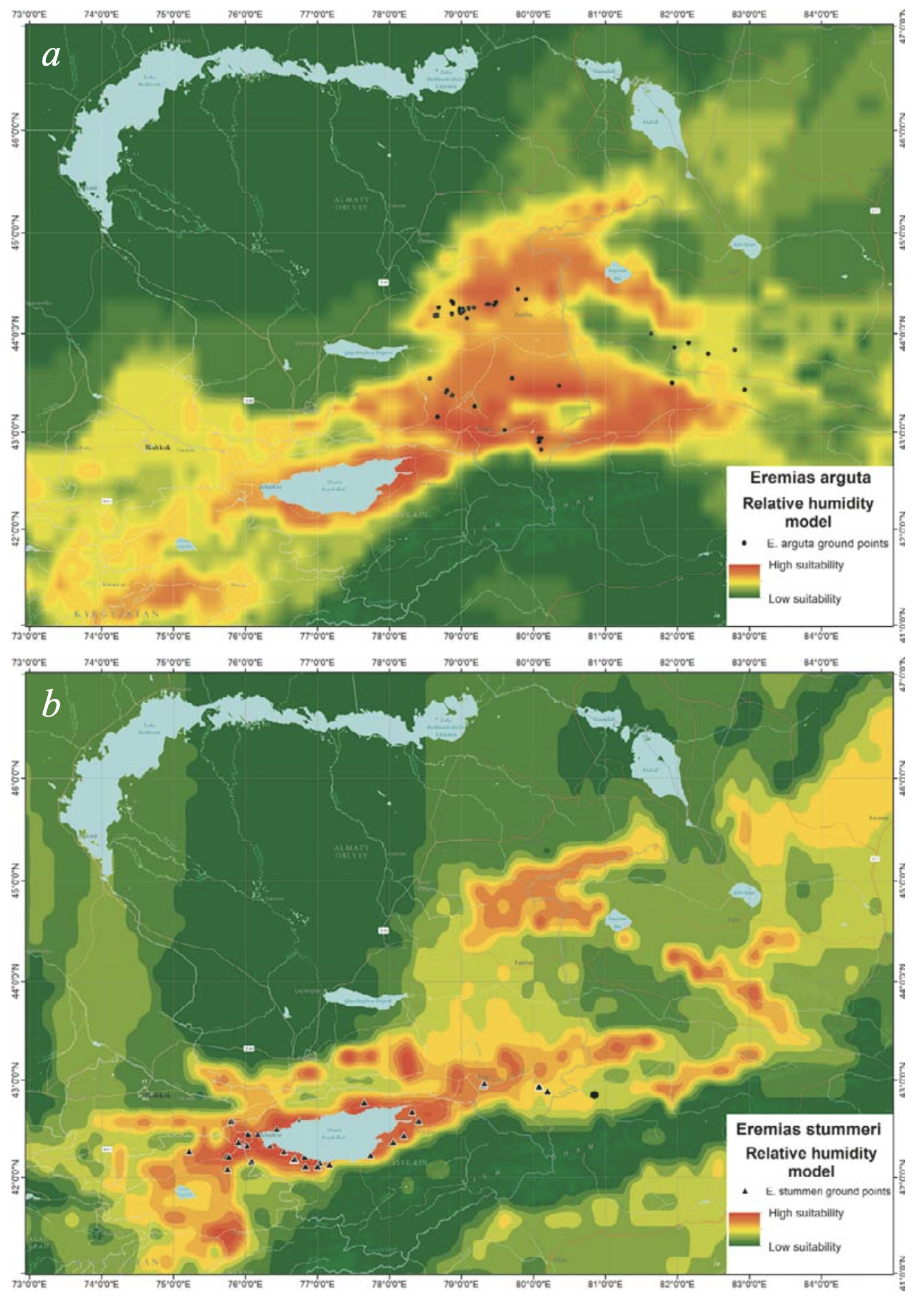
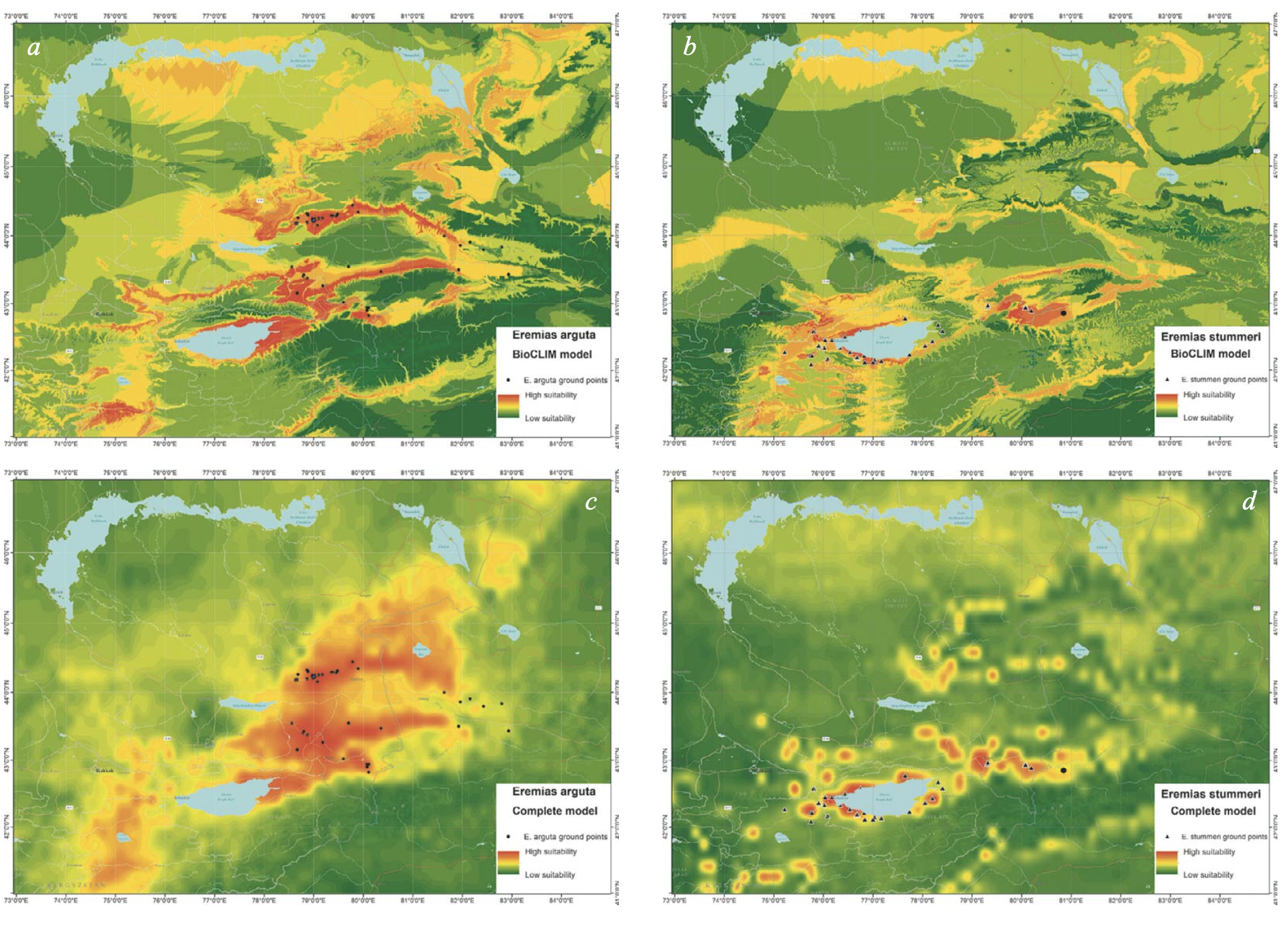
Although differently detailed pictures arose from different spatial resolutions of the input data, the ENMs of E. arguta produced with BIOCLIM and the expanded sets of variables were similar in their basic pattern (Fig. 10a, c). The models show two relatively separated areas which are most suitable for the species. The first area is placed north of the Ily River and includes the Konyrolen Depression, and the southern ridges of the Dzhungarian Alatau and Boro-Khoro within their piedmonts and foothills (see Fig. 3 for the place names). The second area is placed south of the Ily River and includes the piedmonts and foothills of the eastern spurs of Zailiysky Alatau and Ketmen; the Syugaty, Zhalanash, Kegen, and Tekes depressions; and the eastern part of Issyk-Kul Basin. The central part of the Ily Basin looks less suitable for the species. In general, E. arguta distribution model resembles a “horseshoe,” with higher habitat suitability in Kazakhstan compared with those in Xinjiang and Kyrgyzstan.
The ENM of E. stummeri produced with BIOCLIM indicates two relatively continuous areas of greatest suitablity for the species (Fig. 10b). These are the northwestern and southwestern parts of the Issyk-Kul Basin and Boom Canyon in the west; and the Kegen and Tekes depressions (the latter in Kazakhstan alone) in the east (see Fig. 3 for the place names). The Xinjiang part of the Tekes Depression, Kochkor Valley and the eastern part of Issyk-Kul Basin had a lower suitability.
The ENM of E. stummeri produced with the expanded set of variables had a discontinuous pattern (Fig. 10d). A few centers appeared to be most suitable for the species. There were the western, northeastern and southeastern sections of the Issyk-Kul Basin; the Kochkor River Valley; and the Boom Canyon (to a lesser degree), the valleys of Kegen and Tekes rivers and Zhalanash Depression. For some plots in the Matay and Altyn-Emel ridges (the southern spurs of the Dzungarian Alatau); the Katutau Mountains (the northwest part of the Ily Basin); a few limited regions in the Zailiysky Alatau (areas all in Kazakhstan); the Naryn River Valley in Kyrgyzstan; and the Xinjiang part of the Tekes Valley, there was almost 60 – 70% habitat suitability for E. stummeri.
Both patterns of E. arguta ENM were to a large degree determined by precipitation distribution (Fig. 7a); while the patterns of E. stummeri ENM were obviously the sum of the contribution of a number of different variables (see Figs. 7b, 8b, 9b).
DISCUSSION
Our field survey of 2013 – 2016 showed that the “Ily form” E. arguta and E. stummeri are more widely distributed in the contiguous territories of Kazakhstan, Kyrgyzstan and Xinjiang (China) than has been recognized before (Eremchenko and Panfilov, 1999a; Dujsebayeva et al., 2007, 2009), occupying a number of inter-montane depressions (Fig. 3). The field data and ENMs showed that E. arguta and E. stummeri, in general, are quite simi
lar to each other in their ecological preferences, i.e., climate, substrate, landscapes and, to a lesser degree, relief.
Relief and substrates. The DEM described the most suitable habitats of E. arguta as flat or slightly sloping surfaces of zero general curvature for E. arguta; and sloping surfaces of positive general curvature located at 1635 – 2030 m a.s.l. for E. stummeri. E. arguta habitats have lower altitude and lower aspect ranges compared with those of E. stummeri (Table 2). According to geomorphological classification (Edelshtein, 1947; Blaga, 2012), such orographic patterns correspond to the plains, which is the relief preferred by E. arguta; and the rugged terrains (weakly dissected, hilly or mountainous relief) preferred by E. stummeri. As a rule, both species avoided steep slopes.
Flat or slightly sloping and weakly dissected relief and the partial presence of ancient river valleys are typical for the piedmonts and low foothills of Tien Shan inhabited by E. arguta and E. stummeri. There is a transitional zone between the plains and the mountains. In this zone, material eroded from the Pliocene-Pleistocene mountains and transported by proluvial, fluvial, deluvial and alluvial flows was deposited in the foothills as gravelly-pebble conglomerates. A significant percentage of fluvo-glacial sediments and loess deposits originated under climate change in the Late Cenozoic period (Kostenko, 1978a; Grunert and Dasch, 2004; Medeu, 2010; Song et al., 2014; Li et al., 2015). In terms of the paleogeography of other typical representatives in Central Asia, the species of Phrynocephalus guttatus group, the gravelly-pebble conglomerates and powerful loose deposits of the Pliocene-Quaternary were considered as areas of diversification and ways of following the expansion of the ancestor forms of more recent species (Golubev, 1989; Dunayev, 2009). Occasionally, E. stummeri occurred on the soft soils (Orlova et al., 2016; our data for Xinjiang); but these finds were also linked to the loess deposits.
The ENM showed unsuitable conditions for E. arguta in the central part of the Ily Basin (Fig. 10a, c), where the ground consists of the accumulation of aeolian sands of Middle Pleistocene-Holocene alluvial and lacustrine origins (Kostenko, 1970), and partially waterlogged plots of the Ily River floodplain (Lomonovich and Yakovenko, 1963). The field data confirmed the absence of E. arguta in the sands located along the middle flow of Ily River in Kazakhstan (Brushko, 1995; our data) as well as in the Tukai Desert of neighboring Xinjiang (our data). Both species were absent in Kumtekey and Karasaz sites — the unique small sands located in the Kegen Depression. E. stummeri could occasionally penetrate the border of Kumtekey Sands but did not live there. As was mentioned above, E. stummeri did not penetrate the central floodplain parts of the Kegen and Tekes depressions.
In this way, the two species belong to the ecological group of sclerophilic species with a certain bias to petrophilic specialization. They inhabit the mountain piedmonts and low foothills, preferring the plains (E. arguta) or weakly dissected mountainous relief (E. stummeri) with gravelly-pebble conglomerates and powerful loose deposits.
Climate. According to Köppen’s climate classification (Kottek et al., 2006; Peel et al., 2007), the ENMs of both species belong to the cold semi-arid steppe climate zone (BSk: Table 2). The BSk climates tend to be located in the temperate zones or in the continental interiors in some distance from the large bodies of water. The latter statement corresponded well to the geographic position of the “Ily form” of E. arguta and E. stummeri. Their
ranges are located in Central Asia with its sharply continental climate and typical annual temperature range (42 – 46°C) (Vilesov et al., 1986). The area with BSk climates was placed at higher elevations compared with the areas of hot semi-arid climates (BWk); and is characterized by relatively hot and dry summers; cold winters with relatively low snowfall; big diurnal temperature variations; and a lack of precipitation (Kottek et al., 2006; Peel et al., 2007). Such a description corresponds very well to the climate pattern of the habitats suitable to both species (Table 2). The results of ENM confirmed that the E. arguta and E. stummeri populations are linked to the ecological group of xerophilous and moderately heliophilic and thermophilic reptiles or the reptiles of “open xerophilous areas,” to employ the terminology used by Geptner (1945:23). He described such species as the “: <…not true desert forms but which may be termed a steppe form…>” [Geptner (1945:21)]. According to ENM, annual precipitation (BIO12), one of the most important climatic indicators, varies between the desert and steppe climatic ranges for both lizards (Table 1).
The contiguous regions where no one species was found differed visibly in their climatic patterns. The central part of the Ily Basin besides a substrate impropriety (see above) had a typical dry arid mid-latitude climate (BWk: Kottek et al., 2006; Peel et al., 2007) scarce precipitation (less than 100 – 120 mm); multiple predominance of evaporation over precipitation; and high amplitudes of diurnal and seasonal temperatures. The central parts of the Kegen and Tekes depressions presented as wide swampy valleys distinguished by high humidity (Murzayev, 1966; Puziryova, 1975; Medeu, 2010). The upper zone of Northern and Central Tien Shan midland (2000 – 3000 m a.s.l.) was characterized by high annual precipitation (up to 800 mm) and visibly cold summers (8 – 14°C for July) (Gurikov, 1981). In this way, the distribution of the lizards in the mountain piedmonts and low foothills avoids, for E. arguta, the extreme aridity of the neighboring lowland deserts; and, for E. stummeri, the extreme humidity of the central floodplain valleys. The lizard habitats in general correspond to the areas “where climate and vegetation still retain the main features of the desert, but affects the influence of neighboring mountain ranges” (Prozorovsky, 1935:329). Such a climate in Tien Shan at 1000 – 2000 m a.s.l. promotes a development of short or scrubby vegetation with grasses and sparse shrubs and corresponds to the “mountain steppe” landscapes (Glazovskaya, 1953).
Differences in the key variables of ecological niches as an indicator of a species’ fit with a particular ecosystem
In spite of a general similarity in the climatic variables, the ENMs of the two lizards differed markedly in terms of the ENM key variables. Precipitation variables, radiation in the winter and off-season months and postmeridian humidity were the most important for E. arguta habitats; while for the habitats suitable for E. stummeri a number of temperature variables, radiation for most of year and antemeridian humidity were more important (Fig. 5). These types of differences in key climatic variables suggest the way in which these lizards fit with different ecosystems: E. arguta to the arid plains and E. stummeri to the mountains.
Humidity is the main key factor for any arid ecosystem (Odum, 1986). However, humidity is determined by different factors in the flat plains and the rugged terrains (especially in the mountains). The plains are more or less extensive areas of land with almost uniform or poorly dissected relief and relatively weak development of the valleys (Edelshtein, 1947). In the arid plains with their almost flat surfaces, uniform abundant incoming solar radiation and intensive outcoming evaporation, the humidity of the habitat will strongly depend on the precipitation. Walter cited by Odum (1986:268) showed that an annual dry production of vegetation in the deserts of the southwestern Africa is a direct ratio of the annual precipitation. A number of the variables from the precipitation and humidity sets were among the key variables of E. arguta ENM (Fig. 5). A crucial value of these two factors can be seen from the comparison of the total E. arguta ENM and the model layers by precipitation and humidity to a smaller degree (compare Figs. 7a, 9a and 10a, c).
It may be argued that, for E. arguta, unlike the typical populations, which inhabit the wide and open steppe and semi-desert areas (Szczerbak, 1974, 1993; Brushko, 1995), its extreme southeastern populations are attracted to the mountainous areas. However, the almost flat or slightly sloping surface of these areas, with relatively low altitudes (1000 – 1800 m a.s.l.) within low mountain ranges (1500 – 2500 m a.s.l.) (Figs. 2, 4a – c), provides uniform and long-term solar radiation (Fig. 6a). Together with poor precipitation and intensive evaporation, this results in an equable arid warm or hot climate (Murzayev, 1966; Puziryova, 1975; Chen et al., 2010; Sun et al., 2015). The heat associated with the solar radiation is significantly enhanced by the partial openness of these depressions to the strongly heated central part of the Ily Basin and the nearby wide and hot Balkhash Basin. Thus, the piedmonts and low foothills of Tien Shan remain the most suitable habitats for this arid-plain-adapted species.
Poikilothermic animals successfully utilize solar radiation for heating (Shilov, 1985). Among the key variables of E. arguta ENM, the presence of solar radiation in particular months (Fig. 5) corresponds well to the ecology of the species. It provides for the successful exit of the lizard from hibernation (March – April), its breeding season (April – June), the growth of the young before hibernation (September – October), and hibernation itself (November – February) (Brushko, 1995; our data). In the arid lowlands, with low precipitation, humidity reaches its lowest values in the afternoon hours after daily longterm strong heating and intensive evaporation (Uteshev, 1959; Matveyev, 1976). According to E. arguta ENM, afternoon humidity is very important for such vulnerable periods of the lizard’s life such as egg laying, incubation (April – May) and deep hibernation (November – January) (Fig. 5).
Unlike the plains, the mountain landscapes have a dissected relief and fluctuations in height of 200 m and more (Edelshtein, 1947). In the mountains, expression of all the climatic factors is determined by the relief (Barry, 2008; Schiemann et al., 2008). In this case, humidity, rather than precipitation by itself, as a function of the interaction between precipitation, temperature and relief peculiarities, determines the suitability of climate and habitat (Podrezov, 2014). This is well supported by the high similarity of the discrete pattern of E. stummeri ENM completed by the extended set of variables (Fig.10d) and the pattern of the humidity model (Fig. 9b).
The intermontane depressions inhabited by E. stummeri are located at higher altitudes (1600 – 2000 m a.s.l.), have more isolated positions inside the higher mountains (3000 – 4000 m a.s.l.) and have more visibly dissected relief compared with the habitats of E. arguta (Fig. 2; Puziryova, 1975; Shakurov, 1990). The dissected relief determines a shorter and more uneven solar exposition and subsequently incoming solar radiation. The diversity of mesoand microconditions in the mountains significantly affects the local thermic characteristics, which changes even within a short distance at a similar relief and altitude (Gorbunov, 1986). A number of the variables from the radiation and temperature sets expressed in ENM (Fig. 5) indicate the particular importance of these factors for E. stummeri. Yakovleva (1964) noted that the survival of E. multiocellata (= E. stummeri) in the severe climatic conditions of the Central Tien Shan is also primarily due to the intensive solar radiation, which frequently promotes an increase in the temperature of the soil and the lizard’s body. The presence of AM humidity among the key variables of E. stummeri‘s ENM supports the known data on high humidity in the mountains during the AM hours (Uteshev, 1959; Matveyev, 1976), and suggests its retarding influence on the daily and seasonal activity of xerophilous herps (Cherlin, unpublished data; Doronin, personal communication).
A preference by E. stummeri for a wide range of aspects (Table 2) reflects a mosaic distribution of suitable habitats within the complex mountain relief. With that in mind, it can be suggested that E. stummeri has complex adaptive behavior compared with that of E. arguta. To support its physiological activity and to obtain as much heat as possible, E. stummeri is forced to change its spatial position during daily and seasonal activity using curvature peculiarities, different slopes and aspects, in order to find the available habitats. A presence of these orographic parameters among the key variables of E. stummeri ENM correlates well with that assumption (Fig. 5). However, field confirmation would be welcome.
The needs of E. arguta in higher precipitation in winter compared with E. stummeri (Table 2) could be also explained by their different ecosystem accessory. As sclerophylic and petrophylic inhabitants of the area being studied, both lizards do not make deep wintering shelters and hibernate no deeper than 15 – 40 cm beneath the surface (Zimina, 1959; Szczerbak, 1974; Brushko, 1995). Under such conditions, the successful survival of E. arguta, which hibernates in the wide flat plains at low temperatures and frequent strong winds, is possible only under thicker snow; while the mountain microrelief with a diversity of microclimatic niches and an abundance of natural cavities and holes enables the lizard to survive even in snow absence. According to field observation, E. stummeri uses such shelters in winter (Zimina, 1959; Yakovleva, 1964; Szczerbak, 1974).
Some notes on ecological limits in distribution of the two Eremias species
A deep analysis of ENM provides the possibility of determining the ecological limits of species distribution. As has been shown, both species occupy quite similar ecological niches (Table 2), but differ in ENM key variables (Fig. 5) that indicates their different ecosystem accessory. The ecological limits of the distribution of E. arguta and E. stummeri in the region studied become more understandable from this point of view.
As seen from the ENM, the most suitable orographic and climatic conditions for E. arguta are present in the Konyrolen, Syugaty, and Zhalanash depressions, where the lizard is common and numerous (our data). To the east, the continentality of the climate increases and to the east of E79°30¢ the lizard becomes less numerous (Fig. 11: 11 and 14, respectively). The Xinjiang localities are characterized by the highest annual temperature range (BIO7), the lowest annual precipitation (BIO12) and the lowest aridity index (Fig. 11: circles) that demonstrates severe conditions for lizard survival.
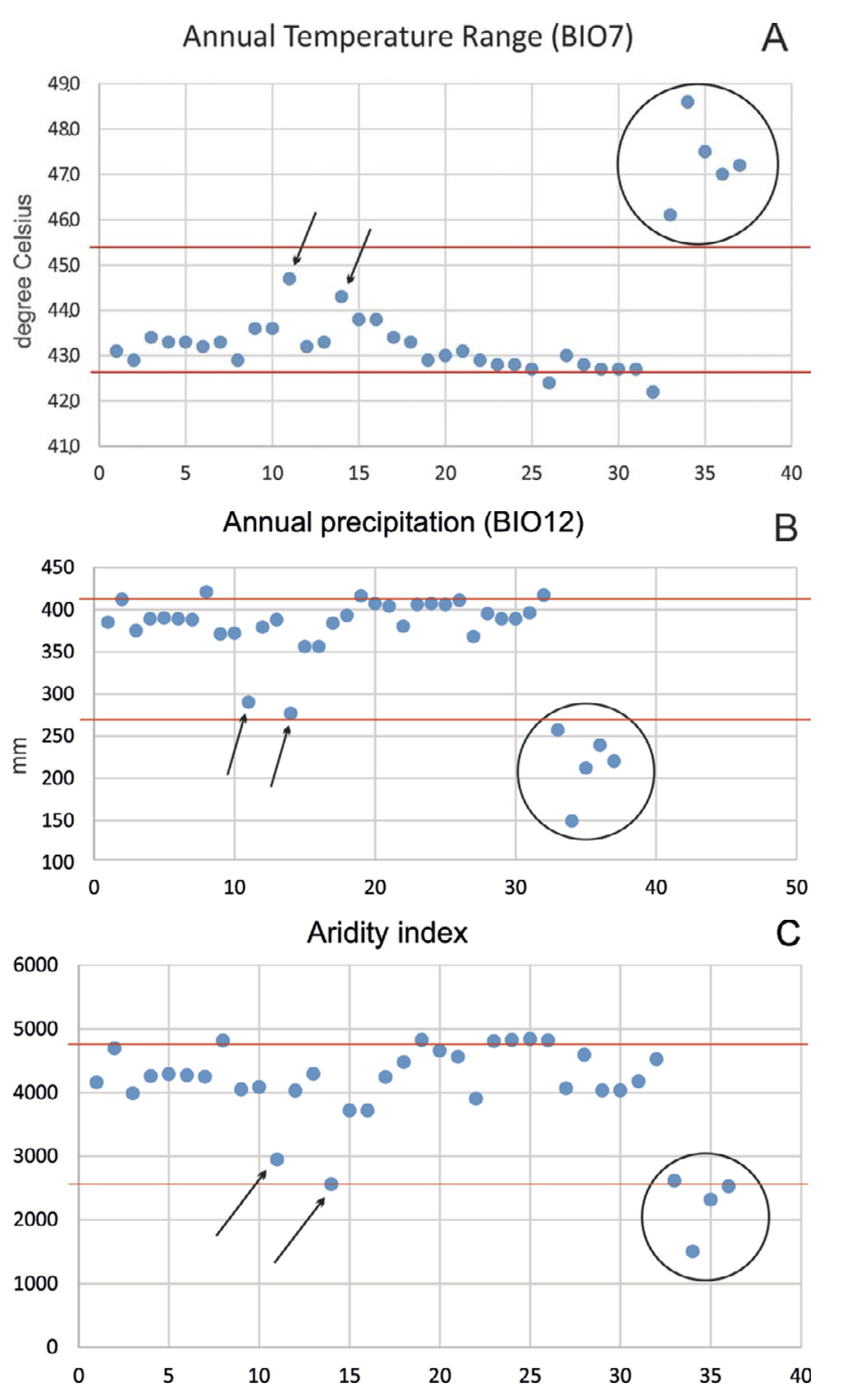
Because of its orographic peculiarities, the Ily Basin differs in its relatively high humidity from other inter-montane basins in Central Asia (Murzayev, 1966).
Nevertheless, the Xinjiang area is visibly drier. The moist westerly Atlantic air masses, moving eastward through the heated Kazakhstan part of the Ily Basin, enter the Xinjiang territory as hot and dry (Lomonovich and Yakovenko, 1963). Obviously, the climate of the intra-montane depressions located further to the east in Xinjiang creates the extreme limit for the distribution of plants and animals (Murzayev, 1966; Abuduvaili and Toropov, 2005). In the extreme eastern regions of the Ily Basin, as well as in the extreme eastern boundary of the species range, E. arguta occurs mainly in the slopingwave foothills with softer microclimate and mountain steppe vegetation that resembles the distribution pattern of Lacerta agilis (Chirikova et al., 2016). The surrounding mountain regions are not suitable for E. arguta as a plain dweller; and the Turpan and Tarim depressions with their extra-continental arid-desert climate are not suitable for E. arguta as a steppe-adapted species.
The extreme southeastern records of E. arguta are registered in the xerophytic Zhabyrtau Mountains where it lives syntopically with E. stummeri (Fig. 3). We did not find E. arguta to the east and south of that site; but based on ENM we suspect its possible presence along the left xerophytic and weakly dissected bank side of the Tekes River in Xinjiang short of the Agiaz River. To the east of the Agiaz, the relief becomes more dissected, with numerous canyons and parent-rock outcrops; and according to ENM there is a better environment for E. stummeri. We confirmed our suggestion by a recent find of E. stummeri in the rangelands with cereals and Achnatherum splendens (Fig. 10b, d: extreme eastern circle).
The ENM showed relatively high suitability for the “Ily form” E. arguta in the northeastern sector of the Issyk-Kul Basin (Fig. 10a, c). This region is inhabited by a separate subspecies, E. a. darevskii, which has some morphological (Tsaruk, 1986; Orlova et al., 2012) and genetic (Poyarkov et al., 2014) peculiarities. However, Chernov (1934) had already noted a significant morphological similarity between the lizards from Issyk-Kul Basin and southern Kazakhstan (Chu and Ily river valleys and Zailiysky Alatau piedmonts — E. a. uzbekistanica Chernov, 1934); and found the specimens of intermediate appearance. In Orlova’s et al. (2012) opinion, the “Ily form” E. arguta in some morphological characters looks like a variation of the E. a. uzbekistanica (= ?E. a. darevskii) subspecies. This fact provides an explanation in the long common history of the relief and biota of the southeastern Kazakhstan and contiguous territories of Kyrgyzstan and Xinjiang (Schultz, 1948; Aleshinskaya et al., 1976; Kostenko, 1978b; Grigina and Fortuna, 1981) and is confirmed by close nestling of all these forms at the base of E. arguta phylogenetic tree (Gong, 2018).
The population of E. stummeri inhabiting the IssykKul Basin is significantly isolated by Kungey and Terskey Alatoo with heights of more than 4000 – 5000 m a.s.l. The highest density of the lizards is seen in the western sector in the landscapes of arid boreal steppes (Shukurov, 1990), where it penetrates north by the Boom Canyon and southwest by the Kochkor River (Yakovleva, 1964; Orlova et al., 2016). Further penetration of the lizards is seemingly limited by the orographic and climatic barriers as well as the presence of a possibly concurrent species — E. arguta in the foothills of the Kyrgyz Range in the north and E. szczerbaki in the Naryn River Valley. E. szczerbaki has been recorded in the Dolon Pass (3030 m a.s.l.) connected the Kochkor and Naryn river valleys (Eremchenko, unpublished data).
The BIOCLIM modeling shows suitable climatic conditions for E. stummeri in the upper flows of the Kegen (local name: Shalkodisu) and the Tekes valleys (Fig. 10b). However, the major part of their floodplains here are swamped. The lizards keep to the drier and hilly places that is why their distributions here have a mosaic pattern. From this perspective, modeling by the expanded dataset gives a more realistic picture (Fig. 10d), and indicates the limited possibility of the standard (19) WorldClim Bioclimatic variables dataset when modeling mountain species.
According to our data, the habitat of E. stummeri near Kegen Town is the most northerly location within the species range. The territories to the north (the northern part of the Kegen Depression and the northern and southern foothills of Ketmen) are probably also quite suitable for this species. However, the greater correspondence of the ecological conditions of these territories to the competitive species E. arguta (Fig. 10a, c vs. 10b, d) and its real wider distribution here, obviously, limit the distribution of E. stummeri.
Zoogeographical notes
Since a discussion of the zoogeographical problems is outside the scope of this paper, we have recorded here only the main comments.
The results of GIS-modeling on the accessory of E. arguta and E. stummeri to different ecosystems also support existing views regarding their origin. Multiple lines of evidence serve as arguments to support an accessory of the E. multiocellata complex to the Central Asian Upland Center of Eurasian biota origin (Geptner, 1936; Szczerbak, 1971), with E. stummeri belonging to the “Tien Shan Center” following Eremchenko et al. (1992); or to the Central Tien Shan Province following the geobotanic division of Rubtsov (1950). These include an association of the ranges of the E. multiocellata complex to the inter-montane depressions of the Tien Shan-Himalayan Highlands (Eremchenko and Panfilov, 1999a; Orlova et al., 2016, 2017; Dujsebayeva et al., 2009); molecular divergence estimates of the basal lineages of that complex as Mid-Late Pliocene (4.2 – 2.6 Ma: Guo et al., 2011; Liu et al., 2019) for conditions of significantly dissected relief (Burtman, 2012; Trifonov et al., 2012); live-birth for the species of E. multiocellata complex (Szscerbak, 1974; Eremchenko et al., 1992; Orlova et al., 1997; Guo et al., 2011); and ENM with temperature and radiation dominating as the key variables. It is very important to emphasize the “mountainous” but not “mountain” adaptation of E. stummeri, compared, for example, with the typical inhabitants of highly dissected mountain areas such as Paralaudakia himalayana (Steindachner, 1867), Asymblepharus eremchenkoi Panfilov, 1999, or the species of Darevskia genus.
Likewise, multiple lines of evidence support the view of Sczcerbak (1971) on the accessory of E. arguta to the “ancient steppe center of Eurasian herpetofauna origin” (p. 53) or to the Kazakhstan center of the “arid steppe and desert plains with hard soils and rare grass” (Geptner, 1945). These include an association of most range areas of E. arguta to the wide lowland plains of Eurasia (Sczcerbak, 1993); molecular divergence estimates of the basal lineages (E. a. uzbekistanica, E. a. darevskii, E. a. ssp.) as Early-Mid Pliocene (4.53 Ma, 95% CI = = 3.06 – 5.89 Ma; Gong, 2018) for the wavy plains of Tien Shan and Dzungarian Alatau (Burtman, 2012; Trifonov et al., 2012); egg-laying (Sczcerbak, 1974, 1993); and ENM with precipitation dominating as the key variable. Based on geobotanic and geomorphologic data, this center has a deep related genetic link with the piedmont and foothill mountain steppes of the Northern Tien Shan (Rubtsov, 1950; Kostenko, 1975).
The only region where E. arguta and E. stummeri were found together was the western part of the Tekes Depression (Fig. 2). Here the lizards were sympatric and syntopic. In the mesophilic stations of the same locality, we found the third lacertid species — Lacerta agilis. Such a species combination with different fauna representatives illustrates the complex history of the Tien Shan biota development. In the Pleistocene, the tectonically dynamic Tien Shan was a territory for the mixing not only of different migratory flows of flora but also different faunas (Geptner, 1936; Rubtsov, 1950; Glazovskaya, 1953).
Paleogeographical notes
The results of ENMs, which describe the dry steppeadapted characteristic of the two Eremias species of different origin, make a feasible contribution to the understanding of the paleogeographic history of the Tien Shan intra-montane depressions.
With the uplifting which began around ~11 – 10 Ma (Abdrakhmatov et al., 2001; Buslov et al., 2004) and continued through the late Miocene-early Pliocene period (~6–3.6Ma), the height of Tien Shan increased to 1000–2000m a.s.l. on average and a maximum of 3200 – 3600 m a.s.l. (Khan-Tengri) (Burtman, 2012; Trifonov et al., 2012). The shallow intra-montane depressions were characterized by a relatively humid climate and the widespread development of lakes and landscapes with woody-shrubby and grassy vegetation without visible stratification (Grigina and Fortuna, 1981; Kostenko, 1978a; Kozhamkulova and Kostenko, 1984). Next came the revival of tectonic activity and the sharp uplift of Tien Shan which took place in the late Pliocene-early Pleistocene, when the mountains almost doubled their height (Kostenko, 1978a; Buslov et al., 2004; Burtman, 2012; Trifonov et al., 2012; Wang et al., 2015). Mountain uplifting was accompanied with further sinking and isolation of the intra-mountain depressions and climate aridification with development of the dry steppe landscapes and xerophilous fauna (Murzayev, 1966; Aleshinskaya and Azikova, 1973; Grigina and Fortuna, 1981).
It seems that the appearance of the ancestral lines of E. arguta and E. stummeri in Tien Shan and their spread within the emerging intra-mountain depressions took place before or at the middle of the Pliocene in the lowland and midland areas of the mountains (Kostenko, 1978a; Burtman, 2012). It is supported by molecular data on separation of the “Ily form” of E. arguta as 3.69 Ma (95% CI = 2.38 – 5.17 Ma; Gong, 2018); and E. stummeri-szczerbakii clade as as 4.69 Ma (95% CI = 3.35 – 6.11 Ma; Liu et al., unpublished data). It also finds confirmation in the generally wide dispersion of the Eremias species within Central Asia in the Pliocene, with a good example as E. velox roborowskii already diverging in the Turpan Basin at approximately 2.99 Ma (95% CI = = 1.59 – 4.51 Ma; Liu et al., 2019). With intense uplifting in the Late Pliocene-Middle Pleistocene, Tien Shan acquired highlands; the intra-montane depressions were isolated by high ridges; and the peripheral areas involved in the uplifting changed the relief from primarily almost horizontal and flat to sloping and dissected (Murzayev, 1966; Trifonov et al., 2012; Wang et al., 2015). At the present time, these areas correspond to the piedmonts and low foothills of the Tien Shan (Kostenko, 1970).
At the point of the subsequent tectonic relief transformation and climate alternations in the Pleistocene, this precise geomorphological zone kept relative stability in terms of climate and landscape. In the growing mountains during the tectonic phases, dissection of the relief intensified. The central parts of the depressions, against the background of the descent of the Neogene lakes (except for the Issyk-Kul), transformed either to the typical deserts (Ily Basin) or to wide mesophilic and marshy floodplains (Karkara, Kegen, and Tekes rivers) (Dzhurkashev, 1972; Grigina and Fortuna, 1981). During the period of glaciation, mountain-valley glaciation developed at a high elevation and the open central lowland areas of the inter-montane and foothill depressions experienced particularly strong cooling with the development of insular permafrost and xerophytic landscapes typical of the tundra-steppes (Aubekerov and Gorbunov, 1999; Yang et al., 2006; Yang and Scuberi, 2010). At the same time, the climate of the piedmont and foothill plains remained relatively stable with less seasonal temperature contrast and less dryness due to increased river runoff from the vast glacial areas and, respectively, more development of the floodplain areas (Serebryanny et al., 1980; Sidikov, 1991; Amosov, 2016).
The visible similarity of the distributional pattern of the two Eremias species in the piedmonts and foothills of Tien Shan with loess strata deposits (Fig. 12) also provides a reason to suggest relative stability of the species ranges during the Pleistocene glaciations. It is known that the environment for sandy loess deposition was generally less arid than the environment developing in the sand seas, and the final stages of loess formation in the Tien Shan were associated with dry steppe vegetation (Glazovskaya, 1953; Fang et al., 2002; Yang et al., 2006). The distribution of E. stummeri in the western sector of the Issyk-Kul Basin lacking the loess accumulations (Fig. 12) can be explained by the geologically recent design of the modern boundaries of the lake basin (Kostenko, 1978a, 197b; Grigina and Fortuna, 1981), rapid development of xerophytic landscapes under abundant radiation, and precipitation deficiency and subsequent lizard penetration from the east.
Finally, according to Abuduvaili and Toropov (2005) for Last Glacial Maximum (LGM), the mean temperatures of January and July in the piedmonts and foothills of Tarim, Dzhungar and Turpan basins were near –(16 – 22)°C and 14 – 16°C, respectively, which seems to be quite close to the minimal temperatures of the coldest months (BIO6) and the mean temperatures of the warmest months (BIO10) of ENMs for both species (Table 2). The annual rainfall in the peripheries of the Xinjiang basins of 20 – 50% preceded the current one that points to less arid ancient conditions, and supports the existence of dry steppes during the LGM even in the very deep regions of Xinjiang. To a greater extent, this could be applied to the Ily Basin and the intra-montane depressions of the Northern and Central Tien Shan with their specific orographic location in Central Asia and milder climate (Murzayev, 1966).
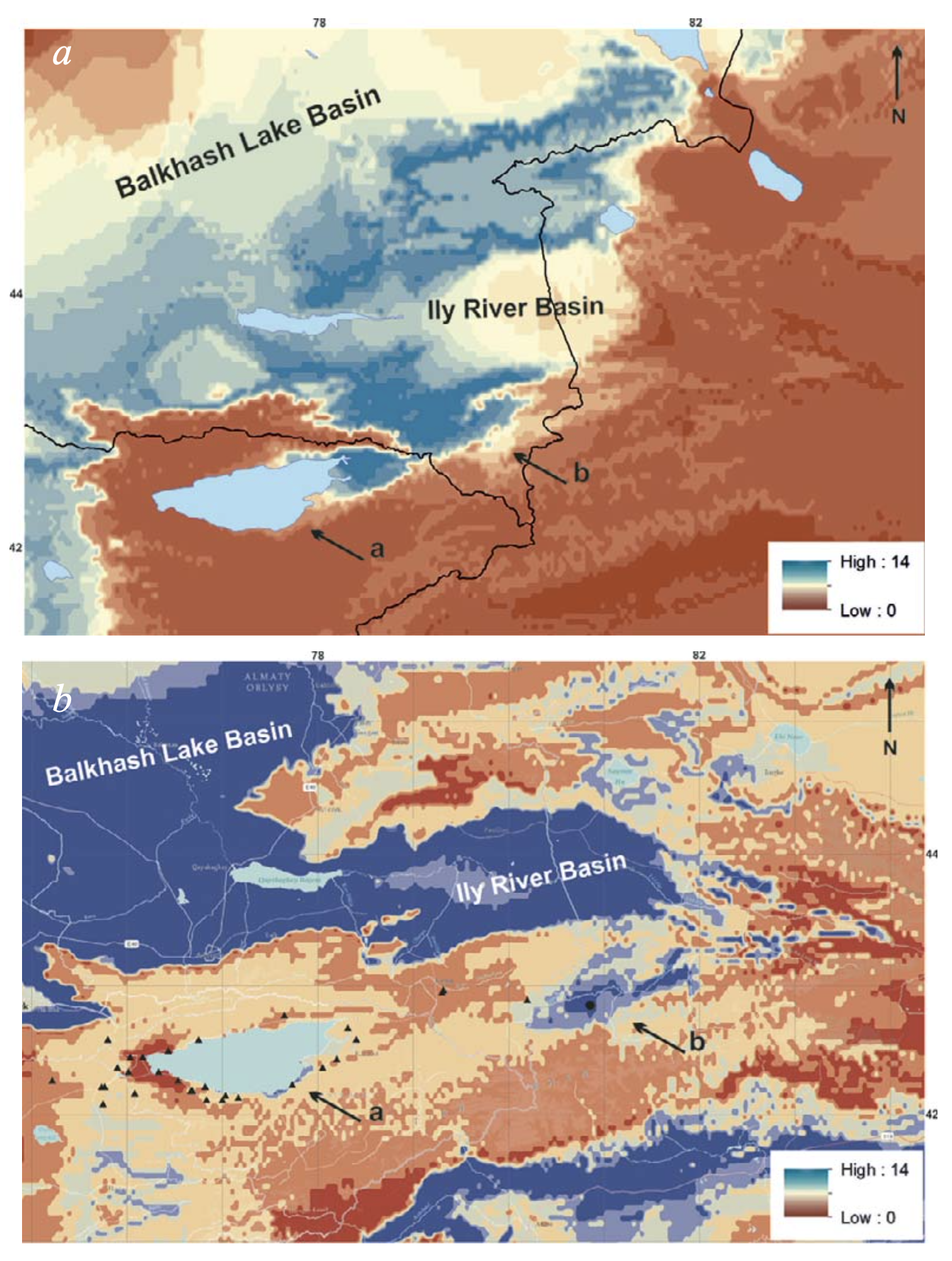
Such arguments imply a relatively suitable environment for E. arguta surviving in the Ily Basin at least during the LGM, and support the view of Poyarkov et al. (2014) on the Ily Basin as a refugium for the arid herpetofauna during the last glaciation. The paleomodel put together for E. arguta has confirmed not very significant changes of the species range during the last glaciation except for the most extreme Xinjiang territory where the species seemingly disappeared (Fig. 10a, c vs. Fig. 13a).
Lizard survival in the harsh LGM conditions of the high mountain regions of Inner and Central Tien Shan seems more problematic. Nevertheless, the paleomodel suggests the Issyk-Kul Basin and Tekes Depression as the possible refugia for E. stummeri during last glaciation (Fig. 13b). These results can be supported by spore-pollen analysis data, indicating an absence of serious changes during the LGM in the landscapes of the piedmonts and foothills of the Inner Tien Shan (Sidikov, 1991) and the Tekes Depression (Aleshinskaya and Azikova, 1973). The Tekes Depression, according to Grigina and Fortuna (1981), obviously served as the refugium for flora also during the preceding glaciations (p. 99). Primary morphological (Orlova et al., 2016) and genetic (Liu et al., unpublished data) differentiation of the Kyrgyz and Kazakhstan populations of E. stummeri confirm the suggestion of two LGM refugia for this species. Some available areas in the Naryn Depression and on the periphery of the Tarim Basin may be potential refugia for other species of the E. multiocellata complex.
CONCLUSIONS
Ecological niche modeling (ENM) opens up wide perspectives for zoological research. Besides many approaches, which were repeatedly demonstrated by numerous recent publications, our results show that ENM provides the possibility to be able to define the ecosystem and zoogeographic accessory of the species that is especially useful for the comparative study of the species with similar ecological preferences, to establish the ecological limits of species distribution, and to restore the paleogeographical sequence of events.
Eremias arguta and E. stummeri belong to the representatives of the arid fauna of Eurasia. Their differentiation and radiation took place in the Late Cenozoic against the backdrop of alpine orogeny and periodic climate alterations with a general trend towards aridization. Both species belong to the ecological group of the xerophilous reptiles, for whom heat and dryness are especially important factors. The optimal ranges of ENM temperature and precipitation mean that these lizards may be considered as “dry-steppe adapted” reptiles. In the mountains of Central Asia, they find suitable habitats in the piedmonts and low foothills with an arid steppe climate (BSk) and mountain steppe landscapes. Inhabiting the piedmonts and low foothills with gravelly-pebble conglomerates and powerful loose deposits, both lizards demonstrate sclerophilous adaptations with a particular bias towards petrophilous specialization.
Despite a general similarity, the ENMs of the two lizards markedly differ in their key variables. Precipitation, radiation during winter and the off-season months and postmeridian humidity were most important for E. arguta; while a number of temperature variables, radiation during most of the year and antemeridian humidity were more important for E. stummeri. The ENMs of E. arguta and E. stummeri also differed in their orography. The most suitable relief for E. arguta was characterized by almost flat or sometimes insignificantly convex and slightly sloping surfaces of mainly western aspects located from over 1058 – 1839 m a.s.l. in height. The most suitable habitats for E. stummeri had a greater curvature, greater slopes, significantly wider aspect and were located at higher altitudes (1635 – 2030 m a.s.l.). Such are the differences in the key climatic and relief variables supporting an accessory of these lizards to different ecosystems: the arid plains for E. arguta and the mountains for E. stummeri. The presence of slope, curvature and aspect among the key variables of E. stummeri ENM supports the idea of more complex adaptive behavior in this species as compared with E. arguta. E. stummeri appears to use the peculiarities of the dissected mountainous relief to support its physiological activity.
The accessory of the two species to different ecosystems, in turn, confirms the views on their zoogeographical origin: the Kazakhstan Center of the arid steppes and deserts for E. arguta and the Tien Shan Center for E. stummeri.
The distributional limits of E. arguta and E. stummeri populations in southeastern Kazakhstan and the surrounding areas of Kyrgyzstan and Xinjiang are determined not only by orographic and hydrological barriers (mountain ranges, river valleys, etc.), but also by the climatic peculiarities of their ecological niches and, in some cases, obviously by the presence of competitive species. The zone of sympatry and syntopy of E. arguta and E. stummeri in the mountains of Zhabyrtau is of special interest from the point of view of species competition.
An analysis of the current distribution of gravel-loess accumulations and the history of their formation might help to clarify the history of radiation and dispersion of xerophilous reptiles in the Late Cenozoic, as has been shown previously for Phrynocephalus guttatus complex. It is assumed that the piedmont and foothill plains of the Tien Shan could serve as the refugia for arid herpetofauna during glacial periods, thus ensuring survival in the absence of long-distance migrations.
Our data indicates the inadequacy of the standard 19 WorldClim Bioclimatic variables dataset in the ecological niche modeling of the mountain species. ENM composed by the expanded set of variables takes into account the leading role of relief in the manifestation of climatic factors (Barry, 2008; Schiemann et al., 2008); and explains the important role that radiation and humidity play in the case of poikilothermic xerophilous mountain species. Our data therefore supports the findings of Beaumont et al. (2005) on the insufficiency of a ‘generalized set’ of parameters in a number of simulation cases. These authors pay attention to the low efficiency of such a set, for example, in a detailed analysis of the ecology of species or “when species are found in different habitat types and bioregions” (p. 265 – 266).
Acknowledgments. We thank Roberto Sindaco (Turin University, Italy) for kind providing us with the records of Eremias stummeri; Alexander Zhdanko (Institute of Zoology, Kazakhstan) for the photo of landscapes of Issyk-Kul Basin; Aldar Gorbunov and Vladimir Shatravin for consultations in LGM events in the territory of the Central and Northern Tien Shan; Ilya Kabak for sharing with his long-term landscape observations in Xinjiang, lizard records and ideas on zoogeographical zoning of Tien Shan and R. Sim for English correction (http:GGwww.expert-english.com). The work was partially funded by grants from Ministry of Education and Sciences of the Republic of Kazakhstan (project No. 1850GGF4), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDPA20050201), and the National Natural Science Foundation of China (31672270).
REFERENCES
- Abdrakhmatov K. E., Weldon R., Thompson S., Burbank D., Rubin Ch., Miller M., and Molnar P. (2001), “Origin, direction, and rate of modern compression in the central Tien Shan, Kyrgyzstan,” Russ. Geol. Geophys., 42, 1585 – 1609.
- Abuduvaili J. and Toropov P. A. (2005), “Variability of landscapes of hollows East Tien-Shan in Holocene and now,” Issl. Zemli Kosm., 5, 63 – 77 [in Russian with English abstract].
- Aleshinskaya Z. V. and Azikova E. K. (1973), “History of vegetation and climate of the Issik-Kul Basin in the Late Pliocene and Pleistocene,” in: Proc. of the III Int. Palinolog. Conf. “Palinology of the Pleistocene and Pliocene,” Nauka, Moscow, pp. 150 – 153 [in Russian].
- Aleshinskaya Z. V., Bondarev L. G., Chigirev N. V., and Shumova G. M. (1976), “About tectonics, climate and glaciation of Tien-Shan in Pleistocene,” in: A. K. Agadzhanyan and O. P. Dobrodeev (eds.), Problems of General Physical Geography and Paleogeography, Izd. MGU, Moscow, pp. 198 – 210 [in Russian].
- Amosov M. I. (2016), “Geographical zones of Eurasian plains during Last Glacial Maximum,” Izv. Rus. Geogr. Obshch., 148(4), 13 – 27 [in Russian with English abstract].
- Ananjev V. P. (1955), “Distribution and composition of loess in the Issyk-Kul (Northern Kyrgyziya),” Dokl. AN KyrgSSR, 101(4), 755 – 758 [in Russian]. Atlas of the Kyrgiz Soviet Socialistic Republic. Vol. 1. Natural Conditions and Resources (1987), Glavnoye Upravlenie Geodezii i Kartografii pri Sovete ministrov USSR, Moscow
- Aubekerov B. and Gorbunov A. P. (1999), “Quaternary permafrost and mountain glaciation in Kazakhstan,” Permafr. Perigl. Processes, 10, 65 – 80.
- Barry R. (2008), Mountain Weather and Climate, 3rd ed., Cambridge Univ. Press, New York.
- Beaumont L. J., Hughes L., and Poulsen M. (2005), “Predicting species distributions: use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions,” Ecol. Model., 186, 250 – 269.
- Bedriaga J. (“1905” 1907), “Verzeichnis der von der CentralAsiatischen Expedition unter Stabs-Kapitän W. Roborowski in den Jahren 1893 – 1895 gesammelten Reptilien,” Ann. Mus. Zool. l’Acad. Imp. Sci. St.-Pétersbourg, 10(3 – 4), 159 – 200.
- Blaga L. (2012), “Aspects regarding the significance of the curvature types and values in the studies of geomorphometry assisted by GIS,” Anal. Univ. Oradea Ser. Geogr., 2012, 327 – 337.
- Burtman V. S. (2012), “Geodynamics of Tibet, Tarim, and the Tien Shan in the Late Cenozoic,” Geotektonika, 3, 18 – 46 [in Russian with English abstract].
- Buslov M. M., De Grave J., and Bataleva E. A. (2004), “Cenozoic tectonics and geodynamic evolution of the Tien Shan mountain belt as response to India-Eurasia convergence,” Himal. J. Sci., 2(4), (extended abstracts: 19th Himalaya – Karakoram – Tibet Workshop, 2004, Niseko, Japan), July 2004), 106 – 107.
- Brushko Z. K. (1995), The Lizards of the Deserts of Kazakhstan, Konzhyk, Almaty [in Russian].
- Chen F., Chen J., Holmes J., Boomer I., Austin P., Gates J. B., Wang N. L., Brooks S. J., and Zhang J. W. (2010), “Moisture changes over the last millennium in arid central Asia: A review, synthesis and comparison with monsoon region,” Quart. Sci. Rev., 29, 1055 – 1068.
- Cherednichenko A. V. (2009), “Climate change in Kazakhstan as a response to its global changing,” Gidrometeorol. Ékol. Almaty, 3, 5 – 14 [in Russian].
- Chernov V. S. (1934), “Über die Unterarten und die Verbreitung von Eremias arguta (Pall.),” Dokl. AN SSSR. Ser. Biol., 3(8 – 9), 666 – 668 [in Russian with German abstract].
- Chirikova M. A., Liu J., and Guo X. (2016), “Zur Verbreitung der Zauneidechse (Lacerta agilis Linnaeus, 1758) an ihrem östlichen Arealrand in Xinjiang, China,” Die Eidechse, 27(1), 15 – 22.
- Coyne J. A. and Orr H. A. (2004), Speciation, Sinauer Associates, Inc., Sunderland, MA.
- Doronin I. V. (2012), “The use of GIS for the analysis of the distribution of Rock Lizards Darevskia (saxicola) complex (Sauria: Lacertidae),” Curr. Stud. Herpetol., 12(3G4), 91 – 122 [in Russian with English abstract].
- Dujsebayeva T. N. and Malakhov D. V. (2017), “The model of Ranodon sibiricus environmental ecological niche: GIS and remotely sensing approach,” Russ. J. Herpetol., 24(3), 171 – 192.
- Dujsebayeva T. N., Chirikova M. A., and Belyalov O. (2009), “New finds of the lizard of Eremias multiocellata-complex in Kazakhstan,” Russ. J. Herpetol., 16(1), 51 – 56.
- Dujsebayeva T. N., Belyalov O., Orlova V. F., and Chirikova M. A. (2007), “Unusual find of the Steppe-Runner, Eremias arguta (Pallas, 1773) with “Ily form” in southeast of Kazakhstan,” Terra, 2, 118 – 121.
- Dunayev E. A. (2009), “Systematics and paleogeography: conceptual synthesis by the example of Phrynocephalus (superspecies guttatus) (Reptilia: Agamidae),” in: A. V. Sviridov and A. I. Shatalkin (eds.), Evolution and Systematics: Lamarck and Darwin in Modern Studies. Archive of the Zoological Museum of Moscow State University. Vol. 50, KMK, Moscow, pp. 275 – 298 [in Russian with English abstract].
- Dzhurkashev T. N. (1972), Anthropogenic History of Balkhsah-Alakol Basin, Nauka, Alma-Ata [in Russian].
- Edelshtein Ya. S. (1947), Basic Geomorphology: Brief Course, 2nd ed., Gos. Izd. Geol. Lit. Min. Geol. USSR, Moscow – Leningrad [in Russian].
- Eremchenko V. K., Panfilov A. M., and Tzarinenko E. I. (1992), “Eremias multiocellata complex: solution of some problems in systematics of the multiocellated racerunners of Kyrgyzstan (Sauria, Lacertidae, Eremias),” in: Abstrs. of the Researches on Cytogenetics and Systematics of Some Asiatic Species of Scincidae and Lacertidae, Ilim, Bishkek, pp. 65 – 80 [in Russian].
- Eremchenko V. K. and Panfilov A. M. (1999a), “Taxonomic situation of multiocellated racerunner of the “multiocellata”-complex of Kyrgyzstan and neighbour China (Sauria: Lacertidae: Eremias),” Nauka Nov. Tekhnol. Ser. Biol., 4, 112 – 124 [in Russian with English abstract].
- Eremchenko V. K. and Panfilov A. M. (1999b), “Taxonomic position and geographic relations of a lacertid lizard Eremias velox from the Issyk-Kul Lake Depression, Tien Shan Mountains, Kyrgyzstan,” Nauka Nov. Tekhnol. Ser. Khim. Biol., 119 – 125 [in Russian with English abstract].
- Escalera-Vázquez L. Y., Hernández-Guzmán R., SotoRojas C., and Suazo-Ortuño I. (2018), “Predicting Ambystoma ordinarium habitat in Central Mexico using Species Distribution Models,” Herpetologica, 74(2), 117 – 126.
- Fang X., Shi Zh., Yang Sh., Yan M., Li J., and Jiang P. (2002), “Loess in the Tian Shan and its implications for the development of the Gurbantunggut Desert and drying of northern Xinjiang,” Chin. Sci. Bull., 47(16), 1381 – 1387.
- Franklin J. (2010), Mapping Species: Distributions. Spatial inference and Prediction, Department of Geography and the Environment, Univ. of Texas, Austin, Texas, USA.
- Geptner V. G. (1936), General Zoogeography, Izd. Biol. Med. Liter., Moscow – Leningrad [in Russian].
- Geptner V. G. (1945), “Desert and steppe fauna of Palearctic regions and centers of its development,” Byull. Obshch. Estestvoispyt. Ser. Biol. Nov. Ser., 50(1 – 2), 17 – 38 [in Russian with English abstract].
- Glazovskaya M. A. (1953), “About the history of development of recent nature landscapes of the Inner Tien-Shan,” in: Geographic Researches in the Central Tien-Shan. Compilation dedicated to memory Semenov-Tyan’-Shansky’s memory, Izd. AN SSSR, Moscow, pp. 27 – 68 [in Russian].
- Golubev M. L. (1989), “Phrynocephalus guttatus (Gmel.) or Ph. versicolor Str. (Reptilia, Agamidae): which Phrynocephalus species occurs in Kazakhstan?” Vestn. Zool., 5, 38 – 46.
- Gong X. (2018), Phylogenetic Systematics and Phylogeography of the Steppe Racerunner (Eremias arguta) in Arid Central Asia. Master Thesis, Univ. Chinese Acad. Sci.
- Gong X., Liu J., Zhou T., Song Q., and Guo X. (2018), “Taxonomical status of Eremias arguta from Bole City and Ili River Valley, Xinjiang Uyghur Autonomous Region, China,” Sichuan J. Zool., 37(4), 387 – 399 [in Chinese with English abstract].
- Gorbunov A. P. (1986), Cryolite Zone of Central Asia, Izd. Inst. Merzlotovedenya SO AN SSSR, Yakutsk [in Russian]. Grant V. (1985), The Evolutionary Process. A Critical Review of Evolutionary Theory, Columbia Univ. Press, New York. Grigina O. M. and Fortuna A. B. (1981), Paleogeography of Northern Tien-Shen through the Cenozoic, Ilim, Frunze [in Russian].
- Grigorjeva E. P. and Zakharov B. S. (1963), “The vegetation of the River Ile middle flow,” in: Ily Valley and Its Natural Resources, Izd. AN KazSSR, Alma-Ata, pp. 103 – 111 [in Russian].
- Groff L. A., Marks S. B., and Hayes M. P. (2014), “Using ecological niche models to direct rare amphibian surveys: a case study using the Oregon Spotted Frog (Rana pretiosa),” Herpetol. Conserv. Biol., 9(2), 354 – 368.
- Grunert J. and Dasch D. (2004), “Dynamics and evolution of dune fields on the northern rim of the Gobi Desert (Mongolia),” Zeitschr. Geomorphol. N. F., 133(Suppl.), 81 – 106.
- Guo X., Dai X., Chen D., Papenfuss T. J., Ananjeva N. B., Melnikov D. A., and Wang Y. (2011), “Phylogeny and divergence times of some racerunner lizards (Lacertidae: Eremias), inferred from mitochondrial 16S rRNA gene segments,” Mol. Phylogenet. Evol., 61, 400 – 412.
- Gurikov D. E. (1981), Zailiysky Alatau, Kainar, Alma-Ata [in Russian].
- Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., and Jarvis A. (2005), “Very high resolution interpolated climate surfaces for global land areas,” Int. J. Climatol., 25, 1965 – 1978.
- Kostenko N. P. (1970), Relief Development of the Highland (on the Example of Middle Asia), Mysl’, Moscow [in Russian].
- Kostenko N. P. (1975), Quaternary Deposits of Highlands, Nedra, Moscow [in Russian].
- Kostenko N. P. (1978a), “The main features of paleogeography of the Late Cenozoic,” in: Quaternary Deposits of Kazakhstan and Adjacent Territories of the Union Republics (Explanatory Note to the Map in Scale 1:1,500,000), Nauka, Alma-Ata, pp. 137 – 146 [in Russian].
- Kostenko N. P. (1978b), “Paleogeography of Issyk-Kul Basin during Cenozoic,” Izv. AN KyrgSSR Ser. Geol., 1, 18 – 25 [in Russian].
- Kottek M., Grieser J., Beck C., Rudolf B., and Rubel F. (2006), “World Map of the Köppen-Geiger climate classification updated,” Meteorol. Zeitschr., 15(3), 259 – 263. Kozhamkulova B. S. and Kostenko N. N. (1984), Ancient Animals of Kazakhstan: (Paleogeography of the Late Cenozoic), Nauka, Alma-Ata [in Russian].
- Li Y., Song Y., Yan L., Chen T., An Z. (2015), “Timing and spatial distribution of loess in Xinjiang, NW China,” PLoS ONE, 10(5), e0125492.
- Liu J., Ananjeva N. B., Chirikova M. A., Milto K. D., and Guo X. (2014), “Molecular assessment and taxonomic status of the Rapid Racerunner (Eremias velox complex) with particular attention to the populations in Northwestern China,” Asian Herpetol. Res., 5(1), 12 – 25.
- Liu J., Guo X., Chen D., Li J., Yue B., and Zeng X. (2019), “Diversification and historical demography of the rapid racerunner (Eremias velox) in relation to geological history and Pleistocene climatic oscillations in arid Central Asia,” Mol. Phylogenet. Evol., 130, 244 – 258.
- Lomonovich M. I. and Yakovenko Z. V. (1963), “Climate,” in: Ily Valley and Its Natural Resources, Izd. AN KazSSR, Alma-Ata, pp. 22 – 39 [in Russian].
- Longley P. A., Goodchild M. F., Maguire D. J., and Rhind D. W. (2005), Geographic Information Systems and Science, John Wiley & Sons.
- Macey R. J., Fong J. J., Kuehl J. V., Shafiei S., Ananjeva N. B., Papenfuss T. J., and Boore J. L. (2005), “The complete mitochondrial genome of a gecko and the phylogenetic position of the Middle Eastern Teratoscincus keyserlingii,” Mol. Phylogenet. Evol., 36, 188 – 193.
- Macey R. J., Schulte J. A., Ananjeva N. B., van Dyke E. T., Wang Y., Orlov N., Shafei S., Robinson M. D., Dujsebayeva T. N., Freund G. S., Fischer C. M., Liu D., and Papenfuss T. J. (2018), “A molecular phylogenetic hypothesis for the Asian agamid lizard genus Phrynocephalus reveals discrete biogeographic clades implicated by plate tectonics,” Zootaxa, 4467(1), 1 – 81.
- Malakhov D. V. and Chirikova M. A. (2018), “Species distribution model of Varanus griseus caspius (Eichwald, 1831) in Central Asia: an insight to the species’ biology,” Russ. J. Herpetol., 25(3), 195 – 206.
- Malakhov D. V., Tsychuyeva N. Yu., and Kambulin V. E. (2018), “Ecological Modeling of Locusta migratoria L. breeding conditions in South-Eastern Kazakhstan,” Russ. J. Ecosyst. Ecol., 3(1).
- Matveyev L. T. (1984), Course of General Meteorology. Physics of the Atmosphere, 2nd Edition, Gidrometeoizdat, Leningrad [in Russian].
- Mayr E. (1966), Animal Species and Evolution, Belknap Press of Harvard Univ. Press, Cambridge, Massachusetts.
- Medeu A. R. (Ed.) (2010), National Atlas of the Republic of Kazakhstan. Vol. 1. Natural Conditions and Resources, 2nd Edition, revised and amended, LLP “Institute of Geography” JSC Nat. Sci. Technol. Holding “Parasat,” Almaty.
- Melville J., Hale J., Mantziou G., Ananjeva N. B., Milto K., and Clemann N. (2009), “Historical biogeography, phylogenetic relationships and intraspecific diversity of agamid lizards in the Central Asian deserts of Kazakhstan and Uzbekistan,” Mol. Phylogenet. Evol., 53, 99 – 112.
- Murzayev E. M. (1966), Nature of Xinjiang and Formation of Central Asian Deserts, Nauka, Moscow
- Odum E. P. (1986), Basic Ecology, CBS College Publ., USA.
- Orlova V. F. and Terbish Kh. (1997), “Family Lacertidae Cope, 1864,” in: Amphibians and Reptiles of Mongolia, KMK Ltd., Moscow, pp. 133 – 266 [in Russian with English abstract].
- Orlova V. F., Chirikova M. A., and Pavlinov I. Ya. (2012), “Steppe Racerunner (Eremias arguta, Sauria, Lacertidae) in the eastern part of its range: morphological variability and taxonomic status of populations,” Zool. Zh., 91(11), 1366 – 1376
- Orlova V. F., Chirikova M. A., Nazarov R. A., and Poyarkov N. A. (2016), “Racerunners of Kyrgyzstan and southeastern part of Kazakhstan (Sauria, Lacertidae, Eremias multiocellata-complex),” Vestn. SPb. Univ. Ser. 3 Biol., 3, 112 – 118 [in Russian with English abstract].
- Orlova V. F., Poyarkov N. A., Chirikova M. A., Nazarov R. A., Munkhbayar M., Munkhbayar Kh., and Terbish Kh. (2017), “MtDNA differentiation and taxonomy of Central Asian racerunners of Eremias multiocellata – E. przewalskii species complex (Squamata, Lacertidae),” Zootaxa, 4282(1), 1 – 42.
- Paraskiv K. P. (1956), Reptiles of Kazakhstan, Izd. AN KazSSR, Alma-Ata [in Russian].
- Peel M. C., Finlayson B. L., and McMahon T. A. (2007), “Updated world map of the Köppen-Geiger climate classification,” Hydrol. Earth Syst. Sci., 11, 1633 – 1644.
- Peterson A. T., Soberón J., Pearson R. G., Anderson R. P., Martínez-Meyer E., Nakamura M., and Araújo M. B. (2011), Ecological Niches and Geographic Distributions, Princeton Univ. Press, New Jersey.
- Podrezov O. A. (2014), Mountain Climatology and Altitudinal Climatic Zonation of Kyrgyzstan, Izd. KyrgGU, Bishkek [in Russian].
- Poyarkov N. A., Orlova V. F., and Chirikova M. A. (2014), “The mitochondrial phylogeography and intraspecific taxonomy of the Steppe Racerunner, Eremias arguta (Pallas) (Lacertidae: Sauria, Reptilia), reflects biogeographic patterns in Middle Asia,” Zootaxa, 3895(2), 208 – 224.
- Prozorovsky A. V. (1935), “About the zonal types of the deserts of Soviet Middle Asia,” Izv. Gos. Geogr. Obshch., 3, 318 – 331 [in Russian].
- Puziryova A. A. (1975), Climatic Zoning of Southern Kazakhstan, Nauka, Alma-Ata [in Russian].
- Rachkovskaya E. I., Volkova E. A., and Khramtsov V. N. (eds.) (2003), Botanical Geography of Kazakhstan and Middle Asia (Desert Region), Boston-Spektra, St. Petersburg.
- Rastegar-Pouyani E., Kazemi Noureini S., Rastegar-Pouyani N., Joger U., and Wink M. (2012), “Molecular phylogeny and intraspecific differentiation of the Eremias velox complex of the Iranian Plateau and Central Asia (Sauria, Lacertidae),” J. Zool. Syst. Evol. Res., 50, 220 – 229.
- Rubtsov N. I. (1950), “On the geobotanical zoning of the Tien Shan,” Byull. Obshch. Estestvoisp. Ser. Biol. Nov. Ser., 55(4), 17 – 38 [in Russian with English abstract].
- Schiemann R., Lüthi D., Vidal P. L., and Schär Ch. (2008), “The precipitation climate of Central Asia — intercomparison of observational and numerical data sources in a remote semiarid region,” Int. J. Climatol., 28, 295 – 314. Schultz S. S. (1948), “Analysis of the Recent Tectonics and the Relief of Tien-Shan,” Zap. Vsesoyuz. Geogr. Obshch. Nov.
- Ser., 3, 1 – 223 [in Russian].
Serebryanny L. R., Pshenin G. N., and Punning Ya.-M. (1980), “Glaciations of the Tien-Shan and fluctuations of the Aral Sea level (staged analysis of the events of Late Quarternary history of Middle Asia),” Izv. AN SSSR Ser. Geogr., 2, 52 – 65 [in Russian]. - Sharaya L. S. and Shary P. A. (2004), “Elementary forms in classifications of relief and their relationship to the characteristics of the landscape of Prioksko-Terrasny Reserve,” Izv. Samar. NT RAN, 1(spec. issue), 102 – 111 [in Russian].
- Shilov I. A. (1985), Physiological Ecology of Animals: Textbook for Students of Biological Specialities of Universities, Vysshaya Shkola, Moscow [in Russian].
- Shnitnikov V. N. (1928), “Reptiles of Semirechye”, Tr. Obshch. Izuch. Kazakhstana [Travaux de la société pour l’étude sur Kazakstan (Pays Kirghiz)], 8(3), 1–85 [in Russian].
- Shukurov E. D. (1990), Ecological and geographical study of Issyk-Kul Depression, Ilim, Frunze
- Sidikov J. (1991), “Paleogeography of Kichi-Naryn River (Inner Tien-Shan) during Late Quaternary and Holocene,” in: Geomorphology and Paleogeography of Issyk-Kul Basin and Inner Tien-Shen, Ilim, Frunze, pp. 38 – 61 [in Russian].
- Sokolov S. I., Assing I. A., Kurmangaliyev A. B., and Serpikov S. K. (1962), Soils of Almaty Oblast, Soils of Kazakhstan. Vol. 4, Izd. AN KazSSR, Alma-Ata [in Russian].
- Solovyeva E. N., Lebedev V. S., Dunayev E. A., Nazarov R. A., Bannikova A. A., Che J., Murphy R. W., and Poyarkov N. A. (2018), “Cenozoic aridization in central Eurasia shaped diversification of toad-headed agamas (Phrynocephalus; Agamidae, Reptilia),” Peer J., 6, e4543.
- Szczerbak N. N. (1971), “Taxonomy of the genus Eremias (Sauria, Reptilia) in connection with the focuses of the desert-steppe fauna development in Palearctic,” Vestn. Zool., 2, 48 – 55 [in Russian with English abstract].
- Szczerbak N. N. (1974), The Racerunners of Palearctic, Naukova dumka, Kiev [in Russian].
- Szczerbak N. N. (1993), “The Area,” in: The Racerunner, Naukova Dumka, Kiev, pp. 9 – 12 [in Russian].
- Song Y., Chen X., Qian L., Li C., Li Y., Li X., Chang H., and An Z. (2014), “Distribution and composition of loess sediments in the Ili Basin, Central Asia,” Quart. Int., 334 – 335, 61 – 73.
- Sun J., Gong Z., Tian Z., Jia Y., and Windley B. (2015), “Late Miocene stepwise aridification in the Asian interior and the interplay between tectonics and climate,” Palaeogeogr. Palaeoclimatol. Palaeoecol., 421, 48 – 59.
- Suzuki N., Olson D. H., and Reilly E. C. (2007), “Developing landscape habitat models for rare amphibians with small geographic ranges: a case study of Siskiyou Mountains salamanders in the western USA,” Biodivers. Conserv., 17(9), 2197 – 2218.
- Tarkhnishvili D., Kaya U., Gavashelishvili A., and Serbinova I. (2008), “Ecological divergence between two evolutionary lineages of the Caucasian salamander: evidence from GIS analysis,” Herpetol. J., 18, 155 – 163.
- Trifonov V. G., Ivanova T. P., and Bachmanov D. M. (2012), “Recent Transformation of the Central Alpine – Himalayan Belt,” Geotektonika, 5, 3 – 21 [in Russian with English abstract].
- Tsaruk O. I. (1986), “Variability of the head pholidosis and interspecific taxonomy of Eremias arguta,” in: Systematics and Ecology of Amphibians and Reptiles. Proc. of the Zool. Inst. Acad. Sci. USSR. Vol. 157, Leningrad, pp. 203 – 214 [in Russian].
- Uteshev A. S. (ed.) (1959), Climate of Kazakhstan, Gidrometeoizdat, Leningrad [in Russian].
- Vilesov E. N., Guzhavina E. A., and Uvarov V. N. (1986), “On the continentality of climate in Kazakhstan,” in: Problems of Hydrology of Irrigable Soils of Kazakhstan, Izd. KazGU, Alma-Ata, pp. 44 – 54 [in Russian].
- Visloguzova A. V., Valdimirov N. M., Gusskova A. I., Medeuov A., Nurmambetov E. I., Potapova G. M., and Sarsakov A. S. (1991), Relief of Kazakhstan (Explanatory Note to the Geomorphological Map of the Kazakh SSR in Scale 1:1,500,000). Part 1, Gylym, Alma-Ata [in Russian].
- WangL.N.,JiJ.Q.,SunD.X.,XuQ.Q.,andHanB.F. (2015), “Chronological constraints on multi-staged rapid cooling of the Tianshan Mountains inferred from apatite fission track analysis of modern river sands,” Sci. China Earth
- Sci., 58, 1305 – 1319. Wielstra B., Crnobrnja-Isailoviæ J., Litvinchuk S., Reijnen B. T., Skidmore A. K., Sotiropoulos K., Toxopeus A. G., Tzankov N., Vukov T., and Arntzen J. W. (2013), “Tracing glacial refugia of Triturus newts based on mitochondrial DNA phylogeography and species distribution modeling,” Front. Zool., 10, 13.
- Yakovleva I. D. (1964), Reptiles of Kirgizstan, Ilim, Frunze [in Russian].
- Yang X. and Scuderi L. A. (2010), “Hydrological and climatic changes in deserts of China since the Late Pleistocene,” Quart. Res., 73, 1 – 9.
- Yang X., Preusser F., and Radtke U. (2006), “Late Quarternary environmental changes in the Taklamakan Desert, western China, inferred from OSL-dated lacustrine and aeolian deposits,” Quart. Sci. Rev., 25, 923 – 932.
- Zimina R. P. (1959), “On the reptiles of Issyk-Kul Depression,” Tr. Inst. Geogr. AN SSSR, 75, 156 – 167 [in Russian]. Zinenko A., Stümpel N., Mazanaeva L., Bakiev A., Shiryaev K., Pavlov A., Kotenko T., Kukushkin O., Chikin Yu., Duisebajeva N., Nilson G., Orlov N., Tuniyev S., Ananjeva N. B., Murphy R. W., and Joger U. (2015), “Mitochondrial phylogeny shows multiply independent ecological transitions and northern dispersion despite of Pleistocene glaciations in meadow and steppe vipers (Vipera ursinii and Vipera renardi),” Mol. Phylogenet. Evol., 84, 85 – 100.
